Energy and our Universe - Pearson Schools
Energy and our Universe - Pearson Schools
Energy and our Universe - Pearson Schools
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
56<br />
(a)<br />
(b)<br />
BTEC’s own res<strong>our</strong>ces<br />
2.10 Producing electrical<br />
energy – batteries<br />
In this section:<br />
Activity A<br />
Write down three appliances you<br />
have used today that are powered<br />
by batteries. Were the batteries<br />
rechargeable or non-rechargeable?<br />
Did you know?<br />
A battery produces electricity by the<br />
chemical reactions that take place<br />
inside it. The chemical inside a battery<br />
is called an electrolyte. Batteries can<br />
be rechargeable or non-rechargeable.<br />
Symbols for (a) a cell <strong>and</strong> (b) a battery.<br />
P6<br />
You have probably used<br />
something powered by a<br />
battery today – y<strong>our</strong> alarm<br />
clock or watch, mp3 player<br />
or a remote control.<br />
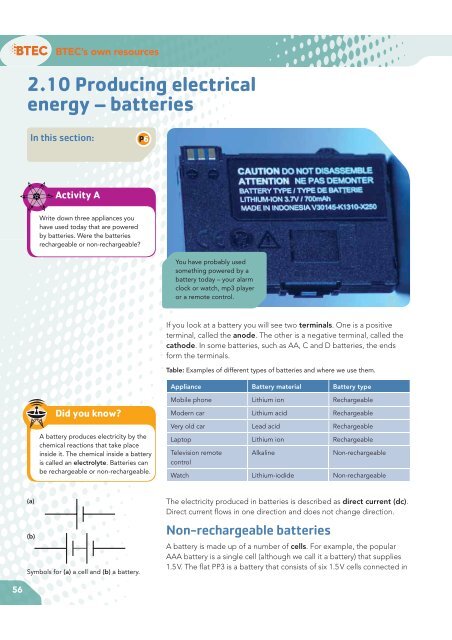
If you look at a battery you will see two terminals. One is a positive<br />
terminal, called the anode. The other is a negative terminal, called the<br />
cathode. In some batteries, such as AA, C <strong>and</strong> D batteries, the ends<br />
form the terminals.<br />
Table: Examples of different types of batteries <strong>and</strong> where we use them.<br />
Appliance Battery material Battery type<br />
Mobile phone Lithium ion Rechargeable<br />
Modern car Lithium acid Rechargeable<br />
Very old car Lead acid Rechargeable<br />
Laptop Lithium ion Rechargeable<br />
Television remote<br />
control<br />
Alkaline Non-rechargeable<br />
Watch Lithium-iodide Non-rechargeable<br />
The electricity produced in batteries is described as direct current (dc).<br />
Direct current fl ows in one direction <strong>and</strong> does not change direction.<br />
Non-rechargeable batteries<br />
A battery is made up of a number of cells. For example, the popular<br />
AAA battery is a single cell (although we call it a battery) that supplies<br />
1.5 V. The fl at PP3 is a battery that consists of six 1.5 V cells connected in