Annex 4 Supplementary guidelines on good manufacturing practices
Annex 4 Supplementary guidelines on good manufacturing practices
Annex 4 Supplementary guidelines on good manufacturing practices
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
140<br />
— standard deviati<strong>on</strong> of the blank; and<br />
— calibrati<strong>on</strong> curve.<br />
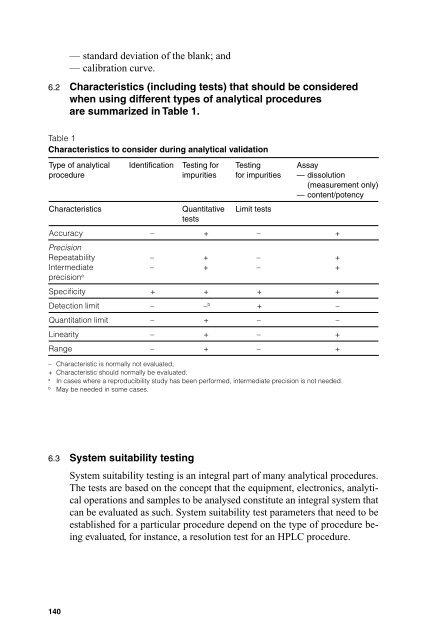
6.2 Characteristics (including tests) that should be c<strong>on</strong>sidered<br />
when using different types of analytical procedures<br />
are summarized in Table 1.<br />
Table 1<br />
Characteristics to c<strong>on</strong>sider during analytical validati<strong>on</strong><br />
Type of analytical<br />
procedure<br />
Identifi cati<strong>on</strong> Testing for<br />
impurities<br />
Characteristics Quantitative<br />
tests<br />
Testing<br />
for impurities<br />
Limit tests<br />
Assay<br />
— dissoluti<strong>on</strong><br />
(measurement <strong>on</strong>ly)<br />
— c<strong>on</strong>tent/potency<br />
Accuracy – + – +<br />
Precisi<strong>on</strong><br />
Repeatability<br />
Intermediate<br />
precisi<strong>on</strong> a<br />
–<br />
–<br />
Specifi city + + + +<br />
Detecti<strong>on</strong> limit – – b + –<br />
Quantitati<strong>on</strong> limit – + – –<br />
Linearity – + – +<br />
Range – + – +<br />
– Characteristic is normally not evaluated;<br />
+ Characteristic should normally be evaluated.<br />
a In cases where a reproducibility study has been performed, intermediate precisi<strong>on</strong> is not needed.<br />
b May be needed in some cases.<br />
6.3 System suitability testing<br />
+<br />
+<br />
System suitability testing is an integral part of many analytical procedures.<br />
The tests are based <strong>on</strong> the c<strong>on</strong>cept that the equipment, electr<strong>on</strong>ics, analytical<br />
operati<strong>on</strong>s and samples to be analysed c<strong>on</strong>stitute an integral system that<br />
can be evaluated as such. System suitability test parameters that need to be<br />
established for a particular procedure depend <strong>on</strong> the type of procedure being<br />
evaluated, for instance, a resoluti<strong>on</strong> test for an HPLC procedure.<br />
–<br />
–<br />
+<br />
+