epoxidation of wild safflower (carthamus oxyacantha) - Research ...
epoxidation of wild safflower (carthamus oxyacantha) - Research ...
epoxidation of wild safflower (carthamus oxyacantha) - Research ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Pawan D. Meshram et al /Int.J. ChemTech Res.2011,3(3) 1154<br />
Iodine monochloride and Wij’s solution were procured from S.D. Fine Chemicals Ltd., Mumbai.<br />
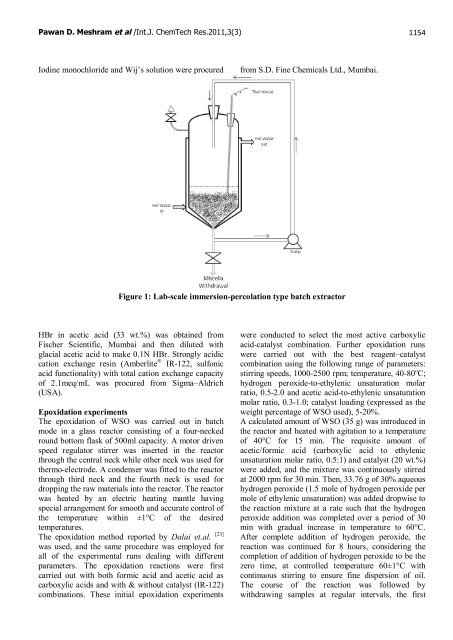
Figure 1: Lab-scale immersion-percolation type batch extractor<br />
HBr in acetic acid (33 wt.%) was obtained from<br />
Fischer Scientific, Mumbai and then diluted with<br />
glacial acetic acid to make 0.1N HBr. Strongly acidic<br />
cation exchange resin (Amberlite ® IR-122, sulfonic<br />
acid functionality) with total cation exchange capacity<br />
<strong>of</strong> 2.1meq/mL was procured from Sigma–Aldrich<br />
(USA).<br />
Epoxidation experiments<br />
The <strong>epoxidation</strong> <strong>of</strong> WSO was carried out in batch<br />
mode in a glass reactor consisting <strong>of</strong> a four-necked<br />
round bottom flask <strong>of</strong> 500ml capacity. A motor driven<br />
speed regulator stirrer was inserted in the reactor<br />
through the central neck while other neck was used for<br />
thermo-electrode. A condenser was fitted to the reactor<br />
through third neck and the fourth neck is used for<br />
dropping the raw materials into the reactor. The reactor<br />
was heated by an electric heating mantle having<br />
special arrangement for smooth and accurate control <strong>of</strong><br />
the temperature within ±1°C <strong>of</strong> the desired<br />
temperatures.<br />
The <strong>epoxidation</strong> method reported by Dalai et.al. [23]<br />
was used, and the same procedure was employed for<br />
all <strong>of</strong> the experimental runs dealing with different<br />
parameters. The <strong>epoxidation</strong> reactions were first<br />
carried out with both formic acid and acetic acid as<br />
carboxylic acids and with & without catalyst (IR-122)<br />
combinations. These initial <strong>epoxidation</strong> experiments<br />
were conducted to select the most active carboxylic<br />
acid-catalyst combination. Further <strong>epoxidation</strong> runs<br />
were carried out with the best reagent–catalyst<br />
combination using the following range <strong>of</strong> parameters:<br />
stirring speeds, 1000-2500 rpm; temperature, 40-80 o C;<br />
hydrogen peroxide-to-ethylenic unsaturation molar<br />
ratio, 0.5-2.0 and acetic acid-to-ethylenic unsaturation<br />
molar ratio, 0.3-1.0; catalyst loading (expressed as the<br />
weight percentage <strong>of</strong> WSO used), 5-20%.<br />
A calculated amount <strong>of</strong> WSO (35 g) was introduced in<br />
the reactor and heated with agitation to a temperature<br />
<strong>of</strong> 40°C for 15 min. The requisite amount <strong>of</strong><br />
acetic/formic acid (carboxylic acid to ethylenic<br />
unsaturation molar ratio, 0.5:1) and catalyst (20 wt.%)<br />
were added, and the mixture was continuously stirred<br />
at 2000 rpm for 30 min. Then, 33.76 g <strong>of</strong> 30% aqueous<br />
hydrogen peroxide (1.5 mole <strong>of</strong> hydrogen peroxide per<br />
mole <strong>of</strong> ethylenic unsaturation) was added dropwise to<br />
the reaction mixture at a rate such that the hydrogen<br />
peroxide addition was completed over a period <strong>of</strong> 30<br />
min with gradual increase in temperature to 60°C.<br />
After complete addition <strong>of</strong> hydrogen peroxide, the<br />
reaction was continued for 8 hours, considering the<br />
completion <strong>of</strong> addition <strong>of</strong> hydrogen peroxide to be the<br />
zero time, at controlled temperature 60±1°C with<br />
continuous stirring to ensure fine dispersion <strong>of</strong> oil.<br />
The course <strong>of</strong> the reaction was followed by<br />
withdrawing samples at regular intervals, the first