Amines & Amides
Amines & Amides
Amines & Amides
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
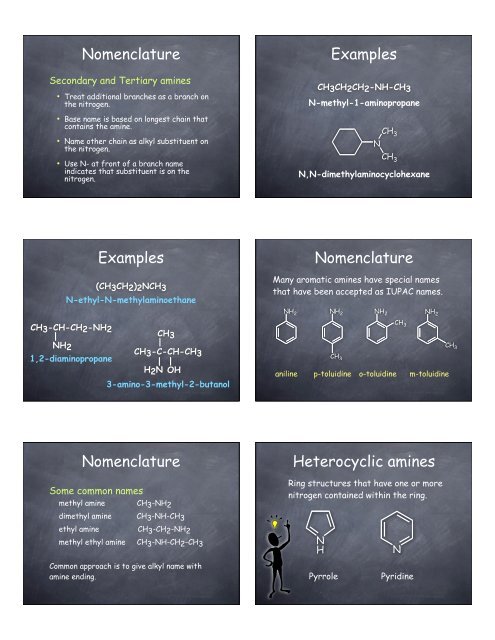
Nomenclature<br />
Secondary and Tertiary amines<br />
• Treat additional branches as a branch on<br />
the nitrogen.<br />
• Base name is based on longest chain that<br />
contains the amine.<br />
• Name other chain as alkyl substituent on<br />
the nitrogen.<br />
• Use N- at front of a branch name<br />
indicates that substituent is on the<br />
nitrogen.<br />
Examples<br />
(CH3CH2)2NCH3<br />
N-ethyl-N-methylaminoethane<br />
CH3-CH-CH2-NH2<br />
|<br />
NH2<br />
1,2-diaminopropane<br />
CH3<br />
CH3-C-CH-CH3<br />
|<br />
H2N OH<br />
3-amino-3-methyl-2-butanol<br />
Nomenclature<br />
Some common names<br />
methyl amine CH 3 -NH 2<br />
dimethyl amine CH 3 -NH-CH 3<br />
ethyl amine CH 3 -CH 2 -NH 2<br />
methyl ethyl amine CH3-NH-CH2-CH3<br />
Common approach is to give alkyl name with<br />
amine ending.<br />
|<br />
|<br />
Examples<br />
CH3CH2CH2-NH-CH3<br />
N-methyl-1-aminopropane<br />
N<br />
CH 3<br />
CH 3<br />
N,N-dimethylaminocyclohexane<br />
Nomenclature<br />
Many aromatic amines have special names<br />
that have been accepted as IUPAC names.<br />
NH 2<br />
NH 2<br />
CH 3<br />
NH 2<br />
CH 3<br />
NH 2<br />
aniline p-toluidine o-toluidine m-toluidine<br />
Heterocyclic amines<br />
Ring structures that have one or more<br />
nitrogen contained within the ring.<br />
N<br />
H<br />
N<br />
Pyrrole Pyridine<br />
CH 3