The advantages of recycling metallic zinc from the ... - Pyrotek
The advantages of recycling metallic zinc from the ... - Pyrotek
The advantages of recycling metallic zinc from the ... - Pyrotek
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Galvanized steel that has been ei<strong>the</strong>r scrapped or recycled is typically just remelted through a<br />
specialized system (quite prevalent in Electric Arc Furnaces) and <strong>the</strong> mill dust and volatized<br />
galvanized coating ash are collected and stored. <strong>The</strong> <strong>zinc</strong> content <strong>of</strong> this mill dust has been<br />
measured to be greater than 20wt% Zn [Ref. 9]. Although a significant amount <strong>of</strong> research has<br />
been devoted toward converting this <strong>zinc</strong>-rich ash into a functional product, <strong>the</strong> results have been<br />
mixed and a consistent, economically viable process has not been widely utilized. On <strong>the</strong> o<strong>the</strong>r<br />
hand, it has been proven that <strong>metallic</strong> <strong>zinc</strong> may be recovered <strong>from</strong> unpainted galvanized steel<br />
using a dedicated electrochemical process [Ref. 10] and <strong>the</strong> practicality <strong>of</strong> <strong>the</strong>se procedures is<br />
now being realized. Thus, only a minimal quantity <strong>of</strong> <strong>metallic</strong> <strong>zinc</strong> is currently recycled <strong>from</strong><br />
galvanized steel substrates.<br />
Conversely, <strong>the</strong> largest source <strong>of</strong> <strong>zinc</strong> waste for galvanizing plants is <strong>zinc</strong> drosses and residues<br />
<strong>from</strong> <strong>the</strong> molten galvanizing bath. Dross forms as a result <strong>of</strong> ei<strong>the</strong>r oxidation <strong>of</strong> <strong>the</strong> <strong>zinc</strong> at <strong>the</strong><br />
bath surface or by inter<strong>metallic</strong> reactions with <strong>the</strong> iron in <strong>the</strong> bath that has been introduced by <strong>the</strong><br />
steel substrates being coated. In <strong>the</strong> two typical categories <strong>of</strong> galvanizing, “general (or batch)<br />
galvanizing” and “continuous galvanizing (CGL)”, <strong>the</strong> <strong>zinc</strong> baths possess variable amounts <strong>of</strong><br />
aluminum (0-0.05wt%Al: General & 0.12-0.3wt%Al: Continuous) resulting in different dross<br />
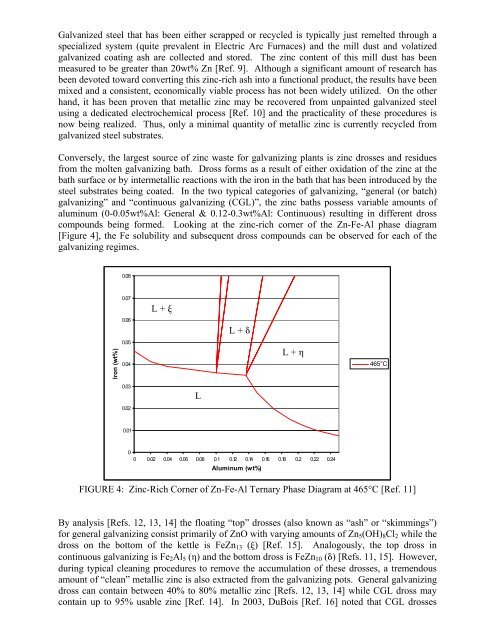
compounds being formed. Looking at <strong>the</strong> <strong>zinc</strong>-rich corner <strong>of</strong> <strong>the</strong> Zn-Fe-Al phase diagram<br />
[Figure 4], <strong>the</strong> Fe solubility and subsequent dross compounds can be observed for each <strong>of</strong> <strong>the</strong><br />
galvanizing regimes.<br />
Iron (wt%)<br />
0.08<br />
0.07<br />
0.06<br />
0.05<br />
0.04<br />
0.03<br />
0.02<br />
0.01<br />
L + ξ<br />
L<br />
L + δ<br />
0<br />
0 0.02 0.04 0.06 0.08 0.1 0.12 0.14 0.16 0.180.2 0.22 0.24<br />
Aluminum (wt%)<br />
L + η<br />
465°C<br />
FIGURE 4: Zinc-Rich Corner <strong>of</strong> Zn-Fe-Al Ternary Phase Diagram at 465°C [Ref. 11]<br />
By analysis [Refs. 12, 13, 14] <strong>the</strong> floating “top” drosses (also known as “ash” or “skimmings”)<br />
for general galvanizing consist primarily <strong>of</strong> ZnO with varying amounts <strong>of</strong> Zn5(OH)8Cl2 while <strong>the</strong><br />
dross on <strong>the</strong> bottom <strong>of</strong> <strong>the</strong> kettle is FeZn13 (ξ) [Ref. 15]. Analogously, <strong>the</strong> top dross in<br />
continuous galvanizing is Fe2Al5 (η) and <strong>the</strong> bottom dross is FeZn10 (δ) [Refs. 11, 15]. However,<br />
during typical cleaning procedures to remove <strong>the</strong> accumulation <strong>of</strong> <strong>the</strong>se drosses, a tremendous<br />
amount <strong>of</strong> “clean” <strong>metallic</strong> <strong>zinc</strong> is also extracted <strong>from</strong> <strong>the</strong> galvanizing pots. General galvanizing<br />
dross can contain between 40% to 80% <strong>metallic</strong> <strong>zinc</strong> [Refs. 12, 13, 14] while CGL dross may<br />
contain up to 95% usable <strong>zinc</strong> [Ref. 14]. In 2003, DuBois [Ref. 16] noted that CGL drosses