CARBONYL COMPOUNDS - CAPE CHEMISTRY
CARBONYL COMPOUNDS - CAPE CHEMISTRY
CARBONYL COMPOUNDS - CAPE CHEMISTRY
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
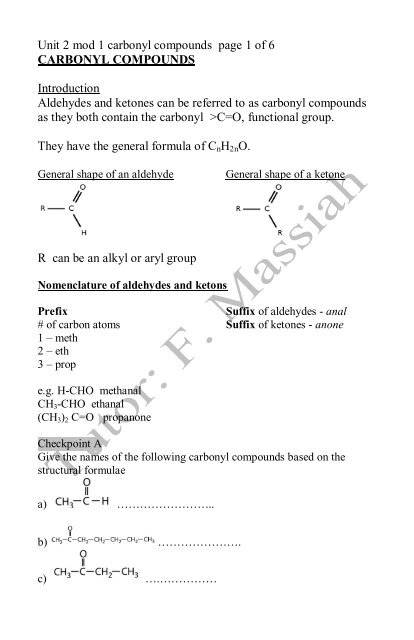
Unit 2 mod 1 carbonyl compounds page 1 of 6<br />
<strong>CARBONYL</strong> <strong>COMPOUNDS</strong><br />
Introduction<br />
Aldehydes and ketones can be referred to as carbonyl compounds<br />
as they both contain the carbonyl >C=O, functional group.<br />
They have the general formula of CnH2nO.<br />
General shape of an aldehyde General shape of a ketone<br />
R can be an alkyl or aryl group<br />
Nomenclature of aldehydes and ketons<br />
Prefix Suffix of aldehydes - anal<br />
# of carbon atoms Suffix of ketones - anone<br />
1 – meth<br />
2 – eth<br />
3 – prop<br />
e.g. H-CHO methanal<br />
CH3-CHO ethanal<br />
(CH3)2 C=O propanone<br />
Checkpoint A<br />
Give the names of the following carbonyl compounds based on the<br />
structural formulae<br />
a) ……………………..<br />
b) ………………….<br />
c) ….……………
Unit 2 mod 1 carbonyl compounds page 2 of 6<br />
How to distinguish between aldehydes and ketones<br />
Reagent Aldehyde Ketone<br />
2,4-DNPH (2,4-dinitrophenylhydrazine) also Yellow or light Yellow or light<br />
called Brady’s reagent<br />
orange ppt<br />
orange ppt<br />
Tollen’s reagent Silver mirror on No visible<br />
inside of test-tube change<br />
Fehling’s reagent Brick-red ppt No ppt formed<br />
Reactions of carbonyl compounds<br />
Reagent Aldehyde Ketone<br />
KMnO4/H + These are oxidised to Ketones are resistant to<br />
carboxylic acids (purple oxidation (remain<br />
to colourless)<br />
purple)<br />
Tollen’s reagent / Aldehydes are oxidised No reaction occurs<br />
Fehling’s reagent and a silver mirror is<br />
seen with Tollen’s<br />
reagent or a brick-red<br />
ppt seen with Fehling’s<br />
reagent<br />
LiAlH4 (ether) These are reduced to the These are reduced to the<br />
primary alcohol secondary alcohol<br />
H2/Pt These are reduced to the These are reduced to the<br />
primary alcohol secondary alcohol<br />
hydrogen cyanide A cyanohydrin or A cyanohydrin or<br />
(NaCN/HCl)<br />
hydroxynitrile is formed hydroxynitrile is formed<br />
Haloform / Iodoform Reaction<br />
This reaction is used to test for the presence of either<br />
The reagents: I2 and NaOH<br />
Observations for positive reaction: fine yellow crystals (iodoform) with a<br />
characteristic “hospital” smell. Iodoform has the formula CHCl3
Unit 2 mod 1 carbonyl compounds page 3 of 6<br />
Checkpoint B
Unit 2 mod 1 carbonyl compounds page 4 of 6<br />
Mechanism for reaction of carbonyl compounds<br />
The mechanism is called nucleophilic addition<br />
The mechanism for the addition of HCN to propanone<br />
In the first stage, there is a nucleophilic attack by the cyanide ion on the<br />
slightly positive carbon atom. This causes a lone pair of electrons to be<br />
repelled towards the oxygen atom forming an ion,<br />
The negative ion would be attracted to a H + ion from the dissociation of<br />
a hydrogen cyanide molecule. NB Since HCN is such a weak acid, this<br />
reaction is usually base catalysed. The resulting molecule is called a<br />
cyanohydrin or a nitrile<br />
The mechanism for the addition of HCN to ethanal<br />
As before, the reaction starts with a nucleophilic attack by the cyanide<br />
ion on the slightly positive carbon atom.<br />
It is completed by the addition of a hydrogen ion from, for example, a<br />
hydrogen cyanide molecule. The resulting molecule is called a<br />
cyanohydrin or a nitrile<br />
Checkpoint C
Unit 2 mod 1 carbonyl compounds page 5 of 6
Unit 2 mod 1 carbonyl compounds page 6 of 6