AusPAR: Cabazitaxel - Therapeutic Goods Administration
AusPAR: Cabazitaxel - Therapeutic Goods Administration
AusPAR: Cabazitaxel - Therapeutic Goods Administration
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Pharmacokinetics<br />
Introduction<br />
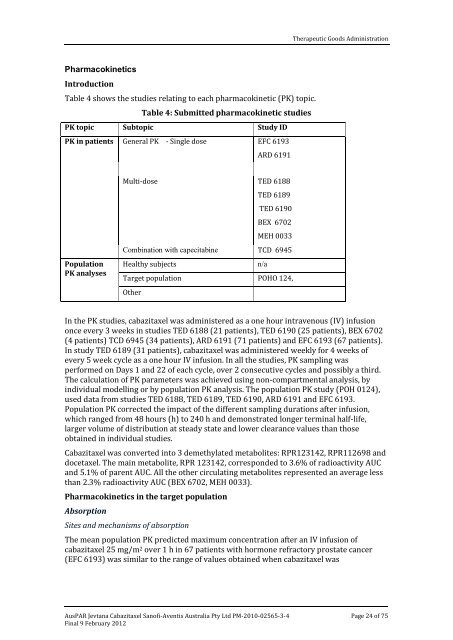
Table 4 shows the studies relating to each pharmacokinetic (PK) topic.<br />
Table 4: Submitted pharmacokinetic studies<br />
PK topic Subtopic Study ID<br />
PK in patients<br />
Population<br />
PK analyses<br />
General PK - Single dose EFC 6193<br />
ARD 6191<br />
Multi-dose TED 6188<br />
TED 6189<br />
TED 6190<br />
BEX 6702<br />
MEH 0033<br />
Combination with capecitabine TCD 6945<br />
Healthy subjects n/a<br />
Target population POHO 124,<br />
Other<br />
<strong>AusPAR</strong> Jevtana <strong>Cabazitaxel</strong> Sanofi-Aventis Australia Pty Ltd PM-2010-02565-3-4<br />
Final 9 February 2012<br />
<strong>Therapeutic</strong> <strong>Goods</strong> <strong>Administration</strong><br />
In the PK studies, cabazitaxel was administered as a one hour intravenous (IV) infusion<br />
once every 3 weeks in studies TED 6188 (21 patients), TED 6190 (25 patients), BEX 6702<br />
(4 patients) TCD 6945 (34 patients), ARD 6191 (71 patients) and EFC 6193 (67 patients).<br />
In study TED 6189 (31 patients), cabazitaxel was administered weekly for 4 weeks of<br />
every 5 week cycle as a one hour IV infusion. In all the studies, PK sampling was<br />
performed on Days 1 and 22 of each cycle, over 2 consecutive cycles and possibly a third.<br />
The calculation of PK parameters was achieved using non-compartmental analysis, by<br />
individual modelling or by population PK analysis. The population PK study (POH 0124),<br />
used data from studies TED 6188, TED 6189, TED 6190, ARD 6191 and EFC 6193.<br />
Population PK corrected the impact of the different sampling durations after infusion,<br />
which ranged from 48 hours (h) to 240 h and demonstrated longer terminal half-life,<br />
larger volume of distribution at steady state and lower clearance values than those<br />
obtained in individual studies.<br />
<strong>Cabazitaxel</strong> was converted into 3 demethylated metabolites: RPR123142, RPR112698 and<br />
docetaxel. The main metabolite, RPR 123142, corresponded to 3.6% of radioactivity AUC<br />
and 5.1% of parent AUC. All the other circulating metabolites represented an average less<br />
than 2.3% radioactivity AUC (BEX 6702, MEH 0033).<br />
Pharmacokinetics in the target population<br />
Absorption<br />
Sites and mechanisms of absorption<br />
The mean population PK predicted maximum concentration after an IV infusion of<br />
cabazitaxel 25 mg/m 2 over 1 h in 67 patients with hormone refractory prostate cancer<br />
(EFC 6193) was similar to the range of values obtained when cabazitaxel was<br />
Page 24 of 75