report on rare disease research, its determinants in ... - Orphanet
report on rare disease research, its determinants in ... - Orphanet
report on rare disease research, its determinants in ... - Orphanet
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
REPORT ON RARE DISEASE RESEARCH, ITS DETERMINANTS IN EUROPE AND THE WAY FORWARD<br />
These market<strong>in</strong>g authorisati<strong>on</strong>s c<strong>on</strong>cern <strong>in</strong>dicati<strong>on</strong>s for the treatment of 66 different RDs and<br />
represent 61 different products.<br />
These figures dem<strong>on</strong>strate that R&D is flourish<strong>in</strong>g <strong>in</strong> the field of RDs but also that therapeutic<br />
developments are far too limited compared to needs.<br />
3.3.2. Determ<strong>in</strong>ants of R&D<br />
3.3.2.1. Prevalence of RDs as a determ<strong>in</strong>ant<br />
One questi<strong>on</strong> we can ask is whether the degree of rarity <strong>in</strong>fluences efforts or even the success of<br />
<strong>research</strong> <strong>in</strong> <strong>rare</strong> <strong>disease</strong>s, as it has been suggested that prevalence directly affects the likelihood of<br />
obta<strong>in</strong><strong>in</strong>g an orphan designati<strong>on</strong> 50<br />
.<br />
When look<strong>in</strong>g at the figures of the COMP (Committee for Orphan Medic<strong>in</strong>al Products) regard<strong>in</strong>g<br />
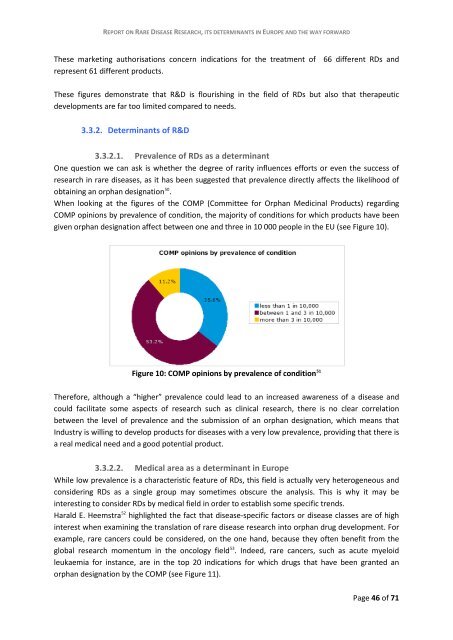
COMP op<strong>in</strong>i<strong>on</strong>s by prevalence of c<strong>on</strong>diti<strong>on</strong>, the majority of c<strong>on</strong>diti<strong>on</strong>s for which products have been<br />
given orphan designati<strong>on</strong> affect between <strong>on</strong>e and three <strong>in</strong> 10 000 people <strong>in</strong> the EU (see Figure 10).<br />
Figure 10: COMP op<strong>in</strong>i<strong>on</strong>s by prevalence of c<strong>on</strong>diti<strong>on</strong> 51<br />
Therefore, although a “higher” prevalence could lead to an <strong>in</strong>creased awareness of a <strong>disease</strong> and<br />
could facilitate some aspects of <strong>research</strong> such as cl<strong>in</strong>ical <strong>research</strong>, there is no clear correlati<strong>on</strong><br />
between the level of prevalence and the submissi<strong>on</strong> of an orphan designati<strong>on</strong>, which means that<br />
Industry is will<strong>in</strong>g to develop products for <strong>disease</strong>s with a very low prevalence, provid<strong>in</strong>g that there is<br />
a real medical need and a good potential product.<br />
3.3.2.2. Medical area as a determ<strong>in</strong>ant <strong>in</strong> Europe<br />
While low prevalence is a characteristic feature of RDs, this field is actually very heterogeneous and<br />
c<strong>on</strong>sider<strong>in</strong>g RDs as a s<strong>in</strong>gle group may sometimes obscure the analysis. This is why it may be<br />
<strong>in</strong>terest<strong>in</strong>g to c<strong>on</strong>sider RDs by medical field <strong>in</strong> order to establish some specific trends.<br />
Harald E. Heemstra 52 highlighted the fact that <strong>disease</strong>-specific factors or <strong>disease</strong> classes are of high<br />
<strong>in</strong>terest when exam<strong>in</strong><strong>in</strong>g the translati<strong>on</strong> of <strong>rare</strong> <strong>disease</strong> <strong>research</strong> <strong>in</strong>to orphan drug development. For<br />
example, <strong>rare</strong> cancers could be c<strong>on</strong>sidered, <strong>on</strong> the <strong>on</strong>e hand, because they often benefit from the<br />
global <strong>research</strong> momentum <strong>in</strong> the <strong>on</strong>cology field 53 . Indeed, <strong>rare</strong> cancers, such as acute myeloid<br />
leukaemia for <strong>in</strong>stance, are <strong>in</strong> the top 20 <strong>in</strong>dicati<strong>on</strong>s for which drugs that have been granted an<br />
orphan designati<strong>on</strong> by the COMP (see Figure 11).<br />
Page 46 of 71