Quiz 1C KEY - Faculty.piercecollege.edu
Quiz 1C KEY - Faculty.piercecollege.edu
Quiz 1C KEY - Faculty.piercecollege.edu
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
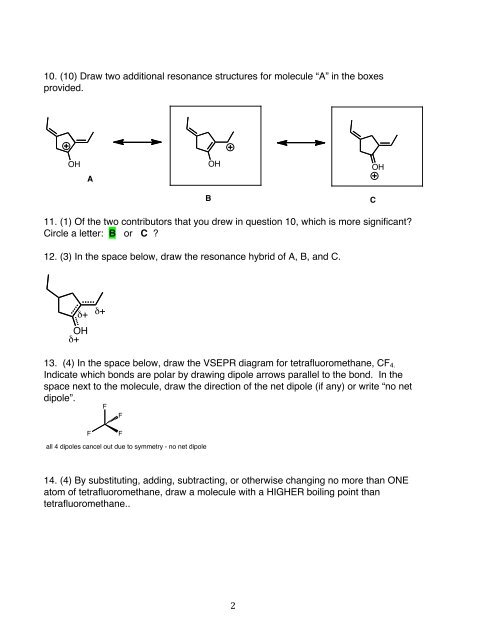
10. (10) Draw two additional resonance structures for molecule “A” in the boxes<br />
provided.<br />
OH OH OH<br />
A<br />
11. (1) Of the two contributors that you drew in question 10, which is more significant?<br />
Circle a letter: B or C ?<br />
12. (3) In the space below, draw the resonance hybrid of A, B, and C.<br />
δ+ δ+<br />
OH<br />
δ+<br />
B C<br />
13. (4) In the space below, draw the VSEPR diagram for tetrafluoromethane, CF4.<br />
Indicate which bonds are polar by drawing dipole arrows parallel to the bond. In the<br />
space next to the molecule, draw the direction of the net dipole (if any) or write “no net<br />
dipole”.<br />
F<br />
F<br />
F F<br />
all 4 dipoles cancel out due to symmetry - no net dipole<br />
14. (4) By substituting, adding, subtracting, or otherwise changing no more than ONE<br />
atom of tetrafluoromethane, draw a molecule with a HIGHER boiling point than<br />
tetrafluoromethane..<br />
2