Empagliflozin for type 2 diabetes mellitus - National Horizon ...
Empagliflozin for type 2 diabetes mellitus - National Horizon ...
Empagliflozin for type 2 diabetes mellitus - National Horizon ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
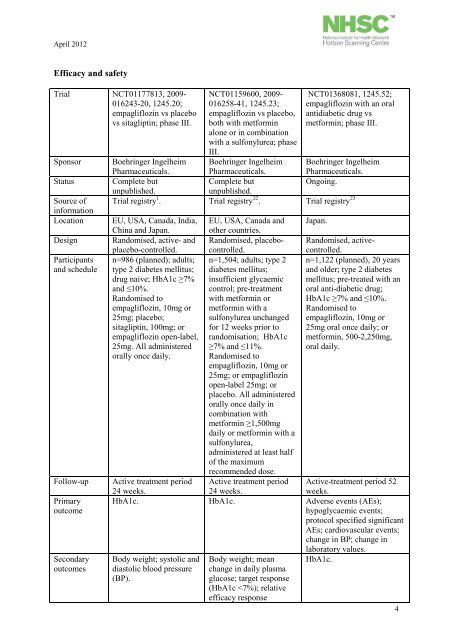
April 2012<br />
Efficacy and safety<br />
Trial NCT01177813, 2009-<br />
016243-20, 1245.20;<br />
empagliflozin vs placebo<br />
vs sitagliptin; phase III.<br />
NCT01159600, 2009-<br />
016258-41, 1245.23;<br />
empagliflozin vs placebo,<br />
both with met<strong>for</strong>min<br />
alone or in combination<br />
with a sulfonylurea; phase<br />
III.<br />
Boehringer Ingelheim<br />
Pharmaceuticals.<br />
Complete but<br />
unpublished.<br />
.<br />
NCT01368081, 1245.52;<br />
empagliflozin with an oral<br />
antidiabetic drug vs<br />
met<strong>for</strong>min; phase III.<br />
Sponsor Boehringer Ingelheim<br />
Boehringer Ingelheim<br />
Pharmaceuticals.<br />
Pharmaceuticals.<br />
Status Complete but<br />
unpublished.<br />
Ongoing.<br />
Source of<br />
in<strong>for</strong>mation<br />
Trial registry 1 . Trial registry 22<br />
23<br />
Trial registry<br />
Location EU, USA, Canada, India, EU, USA, Canada and Japan.<br />
China and Japan. other countries.<br />
Design Randomised, active- and Randomised, placebo- Randomised, active-<br />
placebo-controlled. controlled.controlled.<br />
Participants n=986 (planned); adults; n=1,504; adults; <strong>type</strong> 2 n=1,122 (planned), 20 years<br />
and schedule <strong>type</strong> 2 <strong>diabetes</strong> <strong>mellitus</strong>; <strong>diabetes</strong> <strong>mellitus</strong>; and older; <strong>type</strong> 2 <strong>diabetes</strong><br />
drug naive; HbA1c ≥7% insufficient glycaemic <strong>mellitus</strong>; pre-treated with an<br />
and ≤10%.<br />
control; pre-treatment oral anti-diabetic drug;<br />
Randomised to<br />
with met<strong>for</strong>min or HbA1c ≥7% and ≤10%.<br />
empagliflozin, 10mg or met<strong>for</strong>min with a Randomised to<br />
25mg; placebo;<br />
sulfonylurea unchanged empagliflozin, 10mg or<br />
sitagliptin, 100mg; or <strong>for</strong> 12 weeks prior to 25mg oral once daily; or<br />
empagliflozin open-label, randomisation; HbA1c met<strong>for</strong>min, 500-2,250mg,<br />
25mg. All administered ≥7% and ≤11%.<br />
oral daily.<br />
orally once daily. Randomised to<br />
empagliflozin, 10mg or<br />
25mg; or empagliflozin<br />
open-label 25mg; or<br />
placebo. All administered<br />
orally once daily in<br />
combination with<br />
met<strong>for</strong>min ≥1,500mg<br />
daily or met<strong>for</strong>min with a<br />
sulfonylurea,<br />
administered at least half<br />
of the maximum<br />
recommended dose.<br />
Follow-up Active treatment period Active treatment period Active-treatment period 52<br />
24 weeks.<br />
24 weeks.<br />
weeks.<br />
Primary HbA1c. HbA1c. Adverse events (AEs);<br />
outcome<br />
hypoglycaemic events;<br />
protocol specified significant<br />
AEs; cardiovascular events;<br />
change in BP; change in<br />
laboratory values.<br />
Secondary Body weight; systolic and Body weight; mean HbA1c.<br />
outcomes diastolic blood pressure change in daily plasma<br />
(BP).<br />
glucose; target response<br />
(HbA1c