CDSCO - Guidance for Industry - Central Drugs Standard Control ...
CDSCO - Guidance for Industry - Central Drugs Standard Control ...
CDSCO - Guidance for Industry - Central Drugs Standard Control ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Guidance</strong> <strong>for</strong> <strong>Industry</strong><br />
<strong>Central</strong> <strong>Drugs</strong> <strong>Standard</strong> <strong>Control</strong> Organization Page 48<br />
<br />
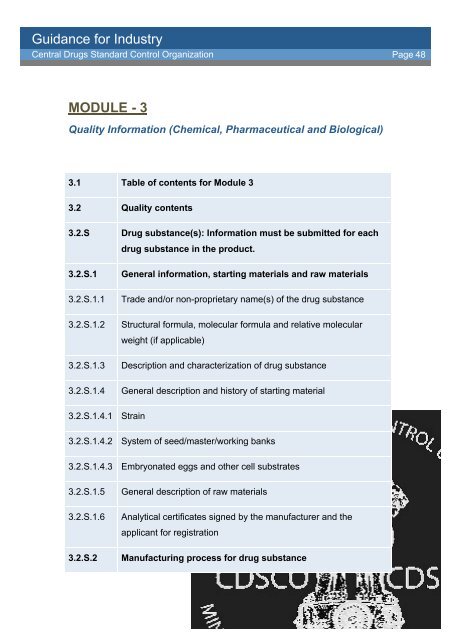
MODULE - 3<br />
Quality In<strong>for</strong>mation (Chemical, Pharmaceutical and Biological)<br />
3.1 Table of contents <strong>for</strong> Module 3<br />
3.2 Quality contents<br />
3.2.S Drug substance(s): In<strong>for</strong>mation must be submitted <strong>for</strong> each<br />
<br />
drug substance in the product.<br />
3.2.S.1 General in<strong>for</strong>mation, starting materials and raw materials<br />
3.2.S.1.1 Trade and/or non-proprietary name(s) of the drug substance<br />
3.2.S.1.2 Structural <strong>for</strong>mula, molecular <strong>for</strong>mula and relative molecular<br />
weight (if applicable)<br />
3.2.S.1.3 Description and characterization of drug substance<br />
3.2.S.1.4 General description and history of starting material<br />
3.2.S.1.4.1 Strain<br />
3.2.S.1.4.2 System of seed/master/working banks<br />
3.2.S.1.4.3 Embryonated eggs and other cell substrates<br />
3.2.S.1.5 General description of raw materials<br />
3.2.S.1.6 Analytical certificates signed by the manufacturer and the<br />
applicant <strong>for</strong> registration<br />
3.2.S.2 Manufacturing process <strong>for</strong> drug substance