4'-(4-Pyridyl)-2,2':6',2"-Terpyridine (Pyterpy) as Ligand in the ...
4'-(4-Pyridyl)-2,2':6',2"-Terpyridine (Pyterpy) as Ligand in the ...
4'-(4-Pyridyl)-2,2':6',2"-Terpyridine (Pyterpy) as Ligand in the ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Global J. Pharmacol., 7 (2): 187-191, 2013<br />
have also been <strong>in</strong>corporated <strong>in</strong>to carbon nanotubes [7]. of <strong>4'</strong>-(4-<strong>Pyridyl</strong>)-2, <strong>2'</strong>:<strong>6'</strong>, 2"-terpyrid<strong>in</strong>e (pyterpy) with<br />
<strong>Terpyrid<strong>in</strong>e</strong> (2,<strong>2'</strong>;<strong>6'</strong>,2"-terpyrid<strong>in</strong>e) is a heterocyclic<br />
1<br />
Gadol<strong>in</strong>ium (III)and identification <strong>the</strong>m with IR, HNMR,<br />
compound derived from pyrid<strong>in</strong>e. This colourless solid TG and DTA<br />
is used <strong>as</strong> a ligand <strong>in</strong> coord<strong>in</strong>ation chemistry.<br />
<strong>Terpyrid<strong>in</strong>e</strong> is a tridentate ligand that b<strong>in</strong>ds metals at<br />
MATERIALS AND METHODS<br />
three meridional sites giv<strong>in</strong>g two adjacent 5-membered<br />
MN2C 2chelate r<strong>in</strong>gs [8]. Gadol<strong>in</strong>ium comb<strong>in</strong>es with most All reagents were supplied by Merck and<br />
elements to form Gd(III) derivatives. nitrogen, carbon, were used without fur<strong>the</strong>r purification. The FT-IR<br />
sulfur, phosphorus, boron, selenium, silicon and spectra were recorded <strong>in</strong> <strong>the</strong> range 400–4000 cm<br />
1<br />
by KBr<br />
arsenic at elevated temperatures, form<strong>in</strong>g b<strong>in</strong>ary disk us<strong>in</strong>g a Bruker Tensor 27 M 420 FT-IR<br />
compounds [9]. <strong>Terpyrid<strong>in</strong>e</strong> forms complexes with spectrophotometer.<br />
most transition metalion <strong>as</strong> do o<strong>the</strong>r polypyrid<strong>in</strong>e<br />
compounds, such <strong>as</strong> 2,<strong>2'</strong>-bipyrid<strong>in</strong>e and 1,10- Syn<strong>the</strong>sis of <strong>the</strong> <strong>4'</strong>-(4-pyridyl)-2, <strong>2'</strong>:<strong>6'</strong>, 2"-terpyrid<strong>in</strong>e<br />
phenanthrol<strong>in</strong>e. Complexes conta<strong>in</strong><strong>in</strong>g two terpyrid<strong>in</strong>e (<strong>Pyterpy</strong>): The ligand pyterpy w<strong>as</strong> prepared by a reported<br />
n+<br />
complexes, i.e. [M(terpy) 2] are common. They differ<br />
1<br />
method [11]. Yield: 85%. M.p. 230-232°C, IR (KBr, /cm ):<br />
n+<br />
structurally from <strong>the</strong> related [M(bipy) 3] complexes <strong>in</strong> 3050 (aromatic C-H stretch), 1475, 1585 (aromatic C=C<br />
be<strong>in</strong>g achiral. <strong>Terpyrid<strong>in</strong>e</strong> complexes, like o<strong>the</strong>r<br />
1<br />
stretch), 1390 (C=N stretch). H NMR (300 MHz, DMSO,<br />
polypyrid<strong>in</strong>e complexes, exhibit characteristic optical and ppm): 7.54 (dd, J = 4.8, 6.15 Hz, 2H, H f), 7.94 (d, J = 4.8 Hz,<br />
electrochemical properties: metal-to-ligand charge transfer 2H, H b), 8.04 (t, J = 7.8 Hz, 2H, H e), 8.68 (d, J = 7.8 Hz,<br />
(MLCT) <strong>in</strong> <strong>the</strong> visible region, reversible reduction and 2H, H g), 8.76 (s, 2H, H c), 8.76-8.77 (4H, H a, H d). UV-Vis<br />
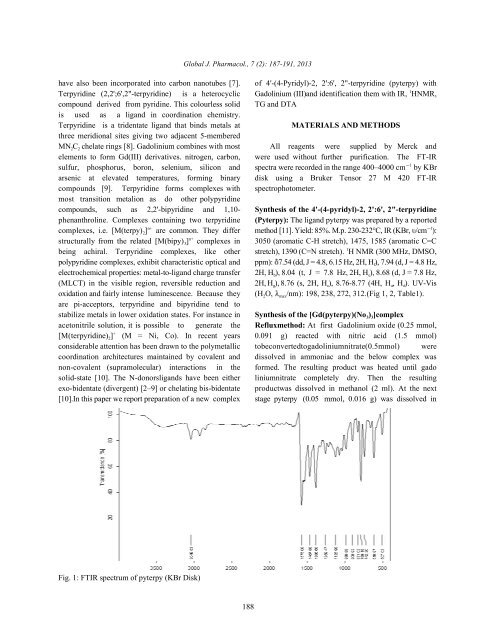
oxidation and fairly <strong>in</strong>tense lum<strong>in</strong>escence. Because <strong>the</strong>y (H2O, max/nm): 198, 238, 272, 312.(Fig 1, 2, Table1).<br />
are pi-acceptors, terpyrid<strong>in</strong>e and bipyrid<strong>in</strong>e tend to<br />
stabilize metals <strong>in</strong> lower oxidation states. For <strong>in</strong>stance <strong>in</strong> Syn<strong>the</strong>sis of <strong>the</strong> [Gd(pyterpy)(No 3) 3]complex<br />
acetonitrile solution, it is possible to generate <strong>the</strong> Refluxmethod: At first Gadol<strong>in</strong>ium oxide (0.25 mmol,<br />
+<br />
[M(terpyrid<strong>in</strong>e) 2] (M = Ni, Co). In recent years 0.091 g) reacted with nitric acid (1.5 mmol)<br />
considerable attention h<strong>as</strong> been drawn to <strong>the</strong> polymetallic tobeconvertedtogadol<strong>in</strong>iumnitrate(0.5mmol) were<br />
coord<strong>in</strong>ation architectures ma<strong>in</strong>ta<strong>in</strong>ed by covalent and dissolved <strong>in</strong> ammoniac and <strong>the</strong> below complex w<strong>as</strong><br />
non-covalent (supramolecular) <strong>in</strong>teractions <strong>in</strong> <strong>the</strong> formed. The result<strong>in</strong>g product w<strong>as</strong> heated until gado<br />
solid-state [10]. The N-donorsligands have been ei<strong>the</strong>r l<strong>in</strong>iumnitrate completely dry. Then <strong>the</strong> result<strong>in</strong>g<br />
exo-bidentate (divergent) [2–9] or chelat<strong>in</strong>g bis-bidentate productw<strong>as</strong> dissolved <strong>in</strong> methanol (2 ml). At <strong>the</strong> next<br />
[10].In this paper we report preparation of a new complex stage pyterpy (0.05 mmol, 0.016 g) w<strong>as</strong> dissolved <strong>in</strong><br />
Fig. 1: FTIR spectrum of pyterpy (KBr Disk)<br />
188