Neurofibromatosis type 1-associated tumours ... - Human Genomics
Neurofibromatosis type 1-associated tumours ... - Human Genomics
Neurofibromatosis type 1-associated tumours ... - Human Genomics
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
REVIEW<br />
Laycock-van Spyk et al.<br />
mutation, even though most exhibit no other NF1<br />
symptoms. 69 Patients may also carry inactivating<br />
mutations of other genes, with a recent study identifying<br />
that 70–80 per cent of mutations involve<br />
genes in the Ras/MAPK pathway, including one<br />
tyrosine-protein phosphatase non-receptor <strong>type</strong> 11<br />
(PTPN11), neuroblastoma RAS viral oncogene<br />
homologue (NRAS), and v-Ki-ras2 kirsten rat<br />
sarcoma viral oncogene homologue (KRAS) as well<br />
as NF1 genes. 70 Additional somatic mutations have<br />
also been reported in the casitas B-lineage lymphoma<br />
(CBL) and additional sex combs-like 1<br />
(ASXL1) genes. 71 In most cases, the NF1 gene is<br />
lost either via LOH or by compound heterozygous<br />
microlesions, 72 which lead to a complete loss of<br />
neurofibromin and hyperactive signalling through<br />
the Ras/MAPK pathway. LOH may occur through<br />
1.2–1.4 Mb interstitial deletions mediated by low<br />
copy number repeat (LCR) elements that flank the<br />
NF1 gene. 73 LOH through uniparental interstitial<br />
isodisomy (50–52.7 Mb) of chromosome 17<br />
through double mitotic recombination, in an<br />
as-yet-unknown initiator cell, has also been<br />
reported. 72 The rarity of such events may indicate<br />
the existence of a selective advantage, conferred<br />
upon the NF1 2/2 cells, which might explain the<br />
propensity of NF1 patients to develop leukaemia. 74<br />
Astrocytomas (ACs)<br />
Optic pathway <strong>tumours</strong> or ACs are found in ≏15<br />
per cent of paediatric NF1 patients, 75 with the<br />
complete loss of neurofibromin evident in<br />
NF1-<strong>associated</strong> optic gliomas. 76 Approximately 84<br />
per cent of NF1-<strong>associated</strong> ACs also exhibit LOH<br />
in the NF1 region, with many <strong>tumours</strong> also exhibiting<br />
LOH of 17p, suggesting the likely role of<br />
TP53 — or other 17p13-located genes — in AC<br />
formation. 77 As with MPNSTs, biallelic somatic<br />
NF1 mutation in ACs is, again, apparently insufficient<br />
to induce transformation.<br />
Phaeochromocytomas (PCs)<br />
PCs are extremely rare <strong>tumours</strong>, with only one to<br />
six cases observed per million individuals. PCs<br />
develop from neural crest-derived chromaffin cells,<br />
and the tumour cells produce and release catecholamines,<br />
which cause hypertension and flushing.<br />
These are <strong>tumours</strong> of the adrenal medulla and<br />
are primarily <strong>associated</strong> with mutations of the<br />
Ret proto oncogene (RET), von Hippel-Lindau<br />
(VHL), succinate dehydrogenase complex, subunit<br />
B (SDHB), succinate dehydrogenase complex,<br />
subunit C (SDHC), and succinate dehydrogenase<br />
complex, subunit D (SDHD) genes, although LOH<br />
in the NF1 region, as well as LOH of other loci on<br />
both 17q and 17p, have been observed. 78,79<br />
Glomus <strong>tumours</strong><br />
Glomus <strong>tumours</strong> are small (,5 mm), benign, but<br />
often very painful <strong>tumours</strong> that develop specifically<br />
within the highly innervated glomus body located<br />
at the end of each digit. These <strong>tumours</strong> appear to<br />
develop from a-smooth muscle actin-positive cells<br />
that have undergone biallelic NF1 inactivation,<br />
resulting in increased Ras/MAPK activity. 80 The<br />
somatic NF1 mutations often differ between<br />
glomus <strong>tumours</strong>, indicating highly specific tumorigenic<br />
events. Brems et al. 80 have suggested that<br />
glomus <strong>tumours</strong>, although rare, should now be<br />
recognised as an integral component of the NF1<br />
spectrum of disease.<br />
The somatic mutational spectrum<br />
of NF1-<strong>associated</strong> <strong>tumours</strong><br />
A review of all published — and the authors’ many<br />
unpublished — somatic NF1 alterations <strong>associated</strong><br />
with NF1 <strong>tumours</strong> was undertaken to gain a better<br />
appreciation of NF1 tumorigenesis. As of July 2010,<br />
at least 577 different somatic NF1 gene changes had<br />
been reported in different NF1-<strong>associated</strong> <strong>tumours</strong>,<br />
with more than half (323/577; 56 per cent) corresponding<br />
to LOH in the NF1 gene region, some<br />
involving much larger regions of chromosome 17<br />
(Table S1). The level of LOH detected also differs<br />
between cutaneous neurofibromas, PNFs and<br />
MPNSTs (40 per cent, 79 per cent and 85 per cent,<br />
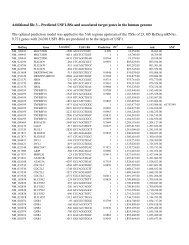
respectively; Table 1). Table 2 provides the incidence<br />
of LOH in the other tumour <strong>type</strong>s, where<br />
628 # HENRY STEWART PUBLICATIONS 1479–7364. HUMAN GENOMICS. VOL 5. NO 6. 623–690 OCTOBER 2011