PDF 816 KB - DOE Hydrogen and Fuel Cells Program Home Page
PDF 816 KB - DOE Hydrogen and Fuel Cells Program Home Page
PDF 816 KB - DOE Hydrogen and Fuel Cells Program Home Page
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Technical Accomplishments <strong>and</strong> Progress<br />
Task 3: Full Length Roll Coating<br />
Anode<br />
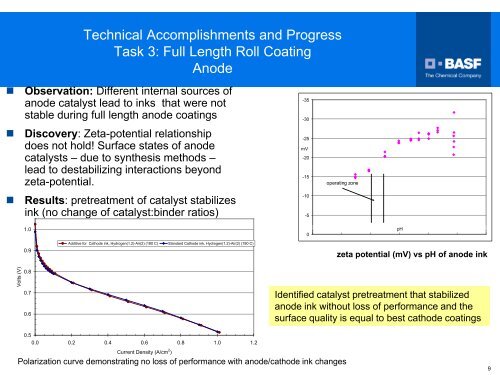
• Observation: Different internal sources of<br />
anode catalyst lead to inks that were not<br />
stable during full length anode coatings<br />
• Discovery: Zeta-potential relationship<br />
does not hold! Surface states of anode<br />
catalysts – due to synthesis methods –<br />
lead to destabilizing interactions beyond<br />
zeta-potential.<br />
• Results: pretreatment of catalyst stabilizes<br />
ink (no change of catalyst:binder ratios)<br />
-35<br />
-30<br />
-25<br />
mV<br />
-20<br />
-15<br />
-10<br />
-5<br />
operating zone<br />
1.0<br />
0.9<br />
Additive for Cathode ink, <strong>Hydrogen</strong>(1.2)-Air(2) (180 C) St<strong>and</strong>ard Cathode ink, <strong>Hydrogen</strong>(1.2)-Air(2) (180 C)<br />
0<br />
0 2 4 6 8 10 12<br />
pH<br />
zeta potential (mV) vs pH of anode ink<br />
Volts (V)<br />
0.8<br />
0.7<br />
0.6<br />
Identified catalyst pretreatment that stabilized<br />
anode ink without loss of performance <strong>and</strong> the<br />
surface quality is equal to best cathode coatings<br />
0.5<br />
0.0 0.2 0.4 0.6 0.8 1.0 1.2<br />
Current Density (A/cm 2 )<br />
Polarization curve demonstrating no loss of performance with anode/cathode ink changes<br />
9