Consultation on Fees to Support EU Biocides Regulation 528 ... - HSE
Consultation on Fees to Support EU Biocides Regulation 528 ... - HSE
Consultation on Fees to Support EU Biocides Regulation 528 ... - HSE
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
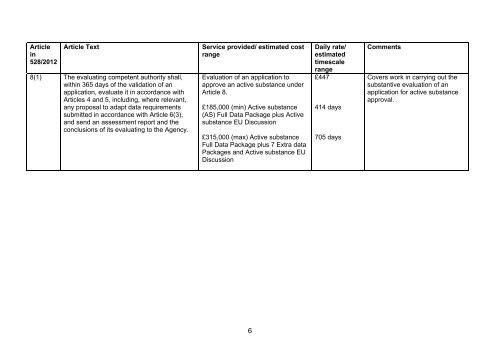
Article<br />
in<br />
<strong>528</strong>/2012<br />
Article Text<br />
8(1) The evaluating competent authority shall,<br />
within 365 days of the validati<strong>on</strong> of an<br />
applicati<strong>on</strong>, evaluate it in accordance with<br />
Articles 4 and 5, including, where relevant,<br />
any proposal <strong>to</strong> adapt data requirements<br />
submitted in accordance with Article 6(3),<br />
and send an assessment report and the<br />
c<strong>on</strong>clusi<strong>on</strong>s of its evaluating <strong>to</strong> the Agency.<br />
Service provided/ estimated cost<br />
range<br />
Evaluati<strong>on</strong> of an applicati<strong>on</strong> <strong>to</strong><br />
approve an active substance under<br />
Article 8.<br />
£185,000 (min) Active substance<br />
(AS) Full Data Package plus Active<br />
substance <strong>EU</strong> Discussi<strong>on</strong><br />
£315,000 (max) Active substance<br />
Full Data Package plus 7 Extra data<br />
Packages and Active substance <strong>EU</strong><br />
Discussi<strong>on</strong><br />
Daily rate/<br />
estimated<br />
timescale<br />
range<br />
£447<br />
414 days<br />
705 days<br />
Comments<br />
Covers work in carrying out the<br />
substantive evaluati<strong>on</strong> of an<br />
applicati<strong>on</strong> for active substance<br />
approval.<br />
6