Rational approach to the selection of conditions for diastereomeric ...
Rational approach to the selection of conditions for diastereomeric ...
Rational approach to the selection of conditions for diastereomeric ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1344 F. C. Ferreira et al. / Tetrahedron: Asymmetry 17 (2006) 1337–1348<br />
Assumptions 5 and 6 are supported by experimental data<br />
published elsewhere. 16 Briefly, this data was obtained by<br />
nan<strong>of</strong>iltering different solutions comprising different molar<br />
ratios <strong>of</strong> diacid resolving agent and racemic PEA, at concentrations<br />
below <strong>the</strong> solubility limits <strong>of</strong> <strong>the</strong> <strong>diastereomeric</strong><br />
salts. The molecular weight cut <strong>of</strong>f <strong>of</strong> <strong>the</strong> membrane employed<br />
was selected in order <strong>to</strong> permeate only <strong>the</strong> unreacted<br />
amine, whilst <strong>the</strong> resolving agent <strong>diastereomeric</strong><br />
salts were retained. The ee <strong>of</strong> <strong>the</strong> permeated solutions<br />
was found <strong>to</strong> be near zero, which confirms Assumption 5.<br />
Moreover, analyses <strong>of</strong> <strong>the</strong> amine in <strong>the</strong> permeate showed<br />
that (i) <strong>the</strong> <strong>for</strong>mation <strong>of</strong> <strong>the</strong> <strong>diastereomeric</strong> salts is practically<br />
irreversible, confirming Assumption 6, and (ii) at molar<br />
ratios between 0.2 and 1.0, neutral salts are <strong>for</strong>med<br />
preferentially <strong>to</strong> acidic salts, confirming <strong>the</strong> species pr<strong>of</strong>iles<br />
obtained in Figure 2a. To expand this data, a similar study<br />
was undertaken <strong>for</strong> <strong>the</strong> PPI2 model system as part <strong>of</strong> <strong>the</strong><br />
present work and similar results were observed (Fig. 7).<br />
There<strong>for</strong>e values <strong>for</strong> <strong>diastereomeric</strong> salts <strong>for</strong>mation constants<br />
(Kd 1 ,Kd 2 ) have been selected at values high enough<br />
<strong>to</strong> simulate irreversible behaviour. Evaluation <strong>of</strong> <strong>the</strong> effects<br />
<strong>of</strong> different values <strong>for</strong> <strong>the</strong>se constants on <strong>the</strong> species pr<strong>of</strong>iles<br />
have been calculated and <strong>the</strong> results <strong>of</strong> <strong>the</strong> models are<br />
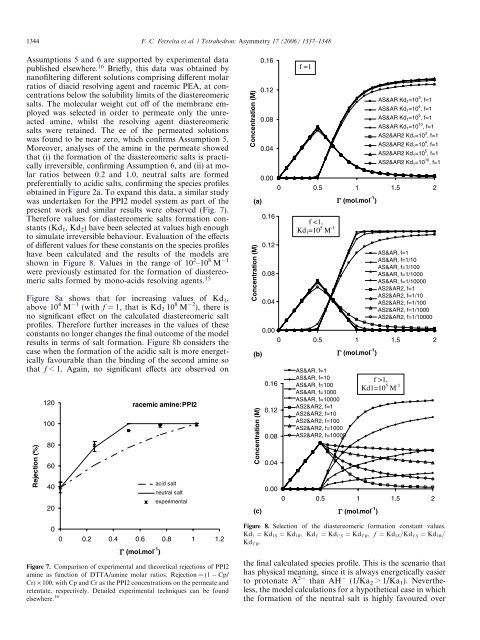
shown in Figure 8. Values in <strong>the</strong> range <strong>of</strong> 10 2 –10 8 M 1<br />
were previously estimated <strong>for</strong> <strong>the</strong> <strong>for</strong>mation <strong>of</strong> <strong>diastereomeric</strong><br />
salts <strong>for</strong>med by mono-acids resolving agents. 15<br />
Figure 8a shows that <strong>for</strong> increasing values <strong>of</strong> Kd 1 ,<br />
above 10 4 M 1 (with f = 1, that is Kd 2 10 8 M 2 ), <strong>the</strong>re is<br />
no significant effect on <strong>the</strong> calculated <strong>diastereomeric</strong> salt<br />
pr<strong>of</strong>iles. There<strong>for</strong>e fur<strong>the</strong>r increases in <strong>the</strong> values <strong>of</strong> <strong>the</strong>se<br />
constants no longer changes <strong>the</strong> final outcome <strong>of</strong> <strong>the</strong> model<br />
results in terms <strong>of</strong> salt <strong>for</strong>mation. Figure 8b considers <strong>the</strong><br />
case when <strong>the</strong> <strong>for</strong>mation <strong>of</strong> <strong>the</strong> acidic salt is more energetically<br />
favourable than <strong>the</strong> binding <strong>of</strong> <strong>the</strong> second amine so<br />
that f < 1. Again, no significant effects are observed on<br />
Rejection (%)<br />
120<br />
100<br />
80<br />
60<br />
40<br />
20<br />
0<br />
racemic amine:PPI2<br />
0 0.2 0.4 0.6 0.8 1 1.2<br />
Γ (mol.mol -1 )<br />
acid salt<br />
neutral salt<br />
experimental<br />
Figure 7. Comparison <strong>of</strong> experimental and <strong>the</strong>oretical rejections <strong>of</strong> PPI2<br />
amine as function <strong>of</strong> DTTA/amine molar ratios. Rejection = (1 Cp/<br />
Cr) · 100, with Cp and Cr as <strong>the</strong> PPI2 concentrations on <strong>the</strong> permeate and<br />
retentate, respectively. Detailed experimental techniques can be found<br />
elsewhere. 16<br />
Concentration (M)<br />
(a)<br />
Concentration (M)<br />
(b)<br />
Concentration (M)<br />
(c)<br />
0.16<br />
0.12<br />
0.08<br />
0.04<br />
0.00<br />
0.16<br />
0.12<br />
0.08<br />
0.04<br />
f =1<br />
0 0.5 1 1.5 2<br />
f 1,<br />
Kd1=10 5 M -1<br />
Figure 8. Selection <strong>of</strong> <strong>the</strong> <strong>diastereomeric</strong> <strong>for</strong>mation constant values.<br />
Kd 1 ¼ Kd 1S ¼ Kd 1R ; Kd 1<br />
0 ¼ Kd 1 0 S ¼ Kd 1 0 R; f ¼ Kd 1S =Kd 1 0 S ¼ Kd 1R =<br />
Kd 1 0 R.<br />
<strong>the</strong> final calculated species pr<strong>of</strong>ile. This is <strong>the</strong> scenario that<br />
has physical meaning, since it is always energetically easier<br />
<strong>to</strong> pro<strong>to</strong>nate A 2 than AH (1/Ka 2 > 1/Ka 1 ). Never<strong>the</strong>less,<br />
<strong>the</strong> model calculations <strong>for</strong> a hypo<strong>the</strong>tical case in which<br />
<strong>the</strong> <strong>for</strong>mation <strong>of</strong> <strong>the</strong> neutral salt is highly favoured over