- Page 1 and 2: Nuclear Science Evaluation of Speci
- Page 3 and 4: ORGANISATION FOR ECONOMIC CO-OPERAT
- Page 5 and 6: OPENING ADDRESS - Satoshi Sakurai,
- Page 7 and 8: SESSION D Methods for Redox Speciat
- Page 9 and 10: Part D: Methods for Redox Speciatio
- Page 12 and 13: EXECUTIVE SUMMARY The Workshop on E
- Page 14: Speciation methods evaluated in the
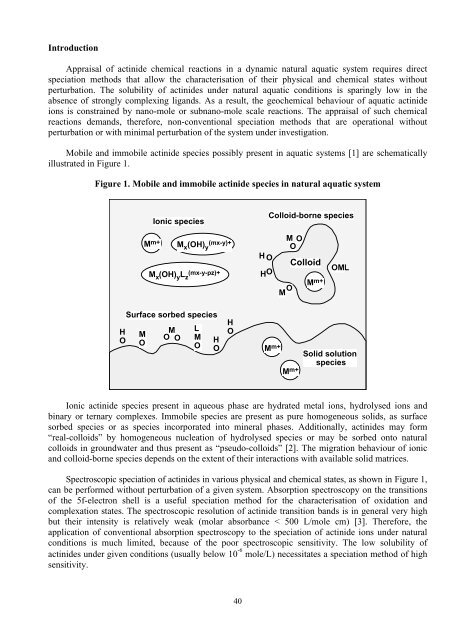
- Page 18 and 19: SPECIATION IMPERATIVES FOR WASTE MA
- Page 20 and 21: matrices poses difficult challenges
- Page 22 and 23: Table 1 lists some of the more comm
- Page 24 and 25: In the complex biosphere there are
- Page 26: SESSION A Methods for Trace Concent
- Page 29 and 30: Introduction Experimental tests of
- Page 31 and 32: complexes. As a result, fulvic acid
- Page 33 and 34: possibility of selective extraction
- Page 35 and 36: Table 1. Data on radiochemical anal
- Page 37 and 38: Figure 1. Flowsheet of isolation of
- Page 42 and 43: A number of spectroscopic methods h
- Page 44 and 45: Figure 4. LPAS and UV-Vis spectra o
- Page 46 and 47: composite and hence it is called br
- Page 48: [11] J.V. Beitz,, J.P. Hessler, “
- Page 52 and 53: SPECIATION FROM PHOTON TO ION DETEC
- Page 54 and 55: technique for speciation (i.e. the
- Page 56 and 57: Figure 2. TRLIF spectra and lifetim
- Page 58 and 59: Figure 5. ES-MS of uranium hydroxo
- Page 60: [11] “Dual use of Micellar Enhanc
- Page 63 and 64: Introduction The solution chemistry
- Page 65 and 66: of the metal sorbed was less than 9
- Page 67 and 68: For the hydrated ions without any c
- Page 69 and 70: those of sorbed Cm(III) and Eu(III)
- Page 71 and 72: Conclusion The luminescence propert
- Page 73 and 74: [34] Graffeo, A.J., Bear, J.L., J.
- Page 75 and 76: Figure 1. Luminescence decay consta
- Page 77 and 78: Figure 3. Plot of the inner-sphere
- Page 79 and 80: Figure 5. Distribution coefficients
- Page 81 and 82: Figure 7. Dissolved fraction of Eu(
- Page 84: SESSION C Methods for Empirical For
- Page 87 and 88: Introduction The application of X-r
- Page 89 and 90:
The mica was left in the solution f
- Page 91 and 92:
That the GIXAS spectra of the Hf(IV
- Page 93 and 94:
[7] Frauenfelder, H., Steffen, R.M.
- Page 95 and 96:
Figure 3. Grazing incidence XAFS be
- Page 97 and 98:
Figure 7. Hf L3 edge GIXAFS spectra
- Page 99 and 100:
Introduction Nuclear magnetic reson
- Page 101 and 102:
ecause there is no longer an anisot
- Page 103 and 104:
20-30 MHz is observed for slowly tu
- Page 105 and 106:
[12] P. Caravan, J.J. Ellison, T.J.
- Page 107 and 108:
Figure 4. NMRD curves of free Gd 3+
- Page 110:
SESSION D Methods for Redox Speciat
- Page 113 and 114:
What is meant by speciation In the
- Page 115 and 116:
• It is non-invasive and non-pert
- Page 117 and 118:
Table 1. Selected absorption bands
- Page 119 and 120:
Figure 3. Shift in absorption edge
- Page 121 and 122:
Introduction Energy production reli
- Page 123 and 124:
then Pu in higher oxidation states
- Page 125 and 126:
• Reliability and reproducibility
- Page 127 and 128:
Conclusion Currently existing techn
- Page 129 and 130:
Table 1. Systems studies; the metho
- Page 132:
SESSION E Predictive Approach to Sp
- Page 135 and 136:
Introduction Thermodynamic data pro
- Page 137 and 138:
secondary mineral formation in immo
- Page 139 and 140:
Additionally, if a phase is impure,
- Page 141 and 142:
REFERENCES [1] T. Murakami, T. Ohnu
- Page 143 and 144:
Figure 1. (a) Backscattered electro
- Page 146 and 147:
CHEMICAL ANALOGY IN THE CASE OF HYD
- Page 148 and 149:
4f-electrons of the lanthanides, an
- Page 150 and 151:
Since water molecules are substitut
- Page 152 and 153:
REFERENCES [1] D. Rai, J.L. Swanson
- Page 154 and 155:
Table 3. Effective charges of actin
- Page 156 and 157:
Figure 3. Atomic number dependence
- Page 158:
POSTER SESSION Part A: Methods for
- Page 161 and 162:
Introduction Several complementary
- Page 163 and 164:
migration. Consequently, only one p
- Page 165 and 166:
Some solutions are under test, such
- Page 168 and 169:
DEVELOPMENT PROGRAMME OF ANALYTICAL
- Page 170 and 171:
Evaluation of the footprints in the
- Page 172 and 173:
REFERENCES [1] B. Pellaud, “IAEA
- Page 174 and 175:
MATRIX-ASSISTED LASER DESORPTION/IO
- Page 176 and 177:
Preparation of the samples There ar
- Page 178 and 179:
Acknowledgement SHIMADZU Handelsges
- Page 180 and 181:
Figure 3. Clusters of uranium(VI) o
- Page 182 and 183:
ASSOCIATION OF ACTINIDES WITH DISSO
- Page 184 and 185:
carried out by the ultra-filters wi
- Page 186 and 187:
REFERENCES [1] N.A. Marley, J.S. Ga
- Page 188 and 189:
Figure 3. Molecular size distributi
- Page 190 and 191:
SPECIATION OF “HOT PARTICLE” BY
- Page 192 and 193:
The clear results were obtained reg
- Page 194 and 195:
in some tens volt per centimetre an
- Page 196 and 197:
APPLICATION OF X-RAY AND LOW ENERGY
- Page 198 and 199:
of 12.5 keV to 23.0 keV. The coeffi
- Page 200 and 201:
[7] R.C. Gatti, H. Nitsche, et al.,
- Page 202:
Figure 1. Calibration results for 2
- Page 205 and 206:
Introduction HQ was used as the ext
- Page 207 and 208:
Iron(III) speciation can be achieve
- Page 209 and 210:
Figure 1. Solubility of HMOnQ in 4
- Page 212 and 213:
SPECIATION OF ENVIRONMENTAL RADIONU
- Page 214 and 215:
precipitate (humic acid fraction) w
- Page 216 and 217:
Acknowledgements This research has
- Page 218 and 219:
Figure 1. Speciation procedure A: e
- Page 220:
POSTER SESSION Part B: Methods for
- Page 223 and 224:
Introduction The possibility of dis
- Page 225 and 226:
fluorescence decay rate of the diff
- Page 227 and 228:
Table 1. Spectroscopic characterist
- Page 229 and 230:
Figure 3. Peak deconvolution of a m
- Page 232 and 233:
SPECIATION ANALYSIS ON EU(III) IN A
- Page 234 and 235:
ACTINYL(VI) SPECIATION IN CONCENTRA
- Page 236 and 237:
Preparation and characterisation of
- Page 238 and 239:
Characterisation of solid AnO2CO3 T
- Page 240 and 241:
Solubility data of uranyl in carbon
- Page 242 and 243:
CHARACTERISATION OF OXIDE FILMS FOR
- Page 244 and 245:
Figure 1. Relative concentration of
- Page 246:
Figure 4. Schematic structure of ox
- Page 249 and 250:
Introduction The actinide elements
- Page 251 and 252:
5LI-(Me-3,2-HOPO (X=CH2 5LIO-(Me-3,
- Page 253 and 254:
Uranium sequestering agents Uranium
- Page 255 and 256:
REFERENCES [1] (a) K.N. Raymond, in
- Page 258 and 259:
SPECIATION OF PU(IV) COMPLEXES WITH
- Page 260 and 261:
will have an absorption spectrum th
- Page 262 and 263:
As shown in our earlier work, Eq (1
- Page 264:
Figure 1. Plots of log Ka-2 D(I) vs
- Page 267 and 268:
As demonstrated above, laser induce
- Page 270:
DIRECT SPECTROSCOPIC SPECIATION OF
- Page 273 and 274:
4 Energy relative to Pu(IV) 3 2 1 0
- Page 275 and 276:
Introduction In R&D on the “back
- Page 277 and 278:
distribution ratios of Am are not s
- Page 279 and 280:
Figure 1. The crystal structure of
- Page 281 and 282:
Table 1. Selected geometrical param
- Page 283 and 284:
Introduction The two important oxid
- Page 285 and 286:
to these two minerals. Ferrihydrite
- Page 287 and 288:
REFERENCES [1] J.K. Osmond and M. I
- Page 290 and 291:
ELECTROANALYTICAL DATA ON URANIUM,
- Page 292 and 293:
- system of Eq. (2), which is measu
- Page 294 and 295:
The following consideration indicat
- Page 296:
Figure 1. Coulopotentiograms for U,
- Page 300 and 301:
SEPARATION AND DETERMINATION OF URA
- Page 302 and 303:
HPEC procedure An aqueous solution
- Page 304 and 305:
REFERENCES [1] T. Braun, G. Ghersin
- Page 306:
Figure 3. Effect of pH on the reten
- Page 309 and 310:
Introduction Voltammetry or polarog
- Page 311 and 312:
voltammograms in Figures 2(a)-2(c)
- Page 313 and 314:
Figure 2. Voltammograms for U(VI) s
- Page 315 and 316:
Introduction One of the most unique
- Page 317 and 318:
The ∆V1/2 of anodic and cathodic
- Page 319 and 320:
Table 1. Half wave potential for ac
- Page 322 and 323:
REDOX SPECIATION OF NP IN TBP EXTRA
- Page 324 and 325:
Analyser). Nitric acid concentratio
- Page 326 and 327:
papers dealing with Np oxidation in
- Page 328 and 329:
Figure 2. Np distribution coefficie
- Page 330 and 331:
Figure 6. Np distribution coefficie
- Page 332:
POSTER SESSION Part E: Predictive A
- Page 335 and 336:
Introduction In the back-end of the
- Page 337 and 338:
CO2 in the solution and were calcul
- Page 339 and 340:
Table 1. Thermochemical cycles for
- Page 341 and 342:
Introduction The essence of data pr
- Page 343 and 344:
(sensitivity analysis). In order to
- Page 345 and 346:
[G15] = LOG(SUM(D15:F15)) (25) Here
- Page 347 and 348:
Table 2. Spreadsheet arrangement of
- Page 349 and 350:
Introduction Scientific studies aim
- Page 351 and 352:
Figure 2. Comparison of non-linear
- Page 353 and 354:
The analysis shows a probability of
- Page 355 and 356:
Internationally accepted QA/QC stan
- Page 358:
SUBGROUP REPORTS 357
- Page 361 and 362:
Advantages On-line CE-MALDI/TOF MS
- Page 363 and 364:
• Colloidal state: - Membrane fil
- Page 365 and 366:
[10] P.A. Bertrand, G.R. Choppin,
- Page 368 and 369:
METHODS FOR MACRO CONCENTRATION SPE
- Page 370 and 371:
Advantages are a complete structure
- Page 372 and 373:
X-ray photoelectron spectroscopy: X
- Page 374 and 375:
The advantages are: • A multi-ele
- Page 376 and 377:
[12] S. Tsutsui, M. Nakada, M. Saek
- Page 378:
[44] H. Capdevila, P. Vitorge, E. G
- Page 381 and 382:
Molecular structure Molecular struc
- Page 383 and 384:
The detection limit of these method
- Page 385 and 386:
By analysing the chemical shifts an
- Page 387 and 388:
Current Chemistry. No. 157, K. Yosh
- Page 390 and 391:
AN OVERVIEW OF ACTINIDE REDOX SPECI
- Page 392 and 393:
Related to this technique is comple
- Page 394 and 395:
Main advantages of the electrochemi
- Page 396 and 397:
Extraction method with use of PMBP,
- Page 398 and 399:
[37] W. Runde, M.P. Neu, S.D. Conra
- Page 400 and 401:
Reagent BaSO4 Silica gel CaCO3 Tabl
- Page 402:
Figure 1. X-ray absorption edge ene
- Page 405 and 406:
The following sections address the
- Page 407 and 408:
priority and are supplemented by do
- Page 409 and 410:
has been addressed, albeit somewhat
- Page 411 and 412:
Table 1: Comparison of •G° f for
- Page 413 and 414:
The full range of environmental par
- Page 415 and 416:
[21] P.L. Brown, R.N. Sylva, “Uni
- Page 418 and 419:
RECOMMENDATION It was recommended t
- Page 420:
There should be allowance for datab
- Page 424 and 425:
Annex 1 LIST OF PARTICIPANTS BELGIU
- Page 426 and 427:
ARISAKA, Makoto Tel: +81 29 282 549
- Page 428 and 429:
NAGAISHI, Ryuji Tel: +81 29 282 549
- Page 430 and 431:
YASUOKA, Hiroshi Tel: +81 29 282 50
- Page 432:
RAI, Dhanpat Tel: +1 509 373 5988 P
- Page 435 and 436:
Participants of the Workshop on Eva
- Page 437 and 438:
ORDER FORM OECD Nuclear Energy Agen