CHEM 11111_2013_05_20 - University of Kelaniya
CHEM 11111_2013_05_20 - University of Kelaniya
CHEM 11111_2013_05_20 - University of Kelaniya
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
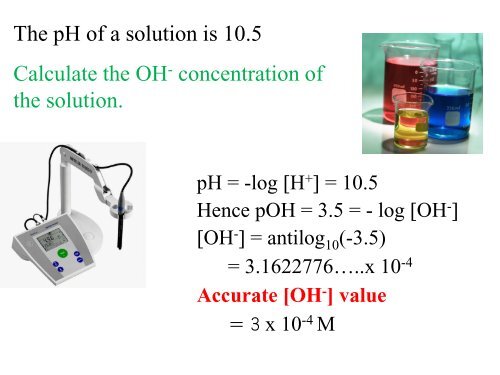
The pH <strong>of</strong> a solution is 10.5<br />
Calculate the OH - concentration <strong>of</strong><br />
the solution.<br />
pH = -log [H + ] = 10.5<br />
Hence pOH = 3.5 = - log [OH - ]<br />
[OH - ] = antilog 10 (-3.5)<br />
= 3.1622776…..x 10 -4<br />
Accurate [OH - ] value<br />
= 3 x 10 -4 M