EY-Global-Forensic-Data-Analytics-Survey-2014
EY-Global-Forensic-Data-Analytics-Survey-2014
EY-Global-Forensic-Data-Analytics-Survey-2014
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Big risks require big data thinking<br />
<strong>Global</strong> <strong>Forensic</strong> <strong>Data</strong> <strong>Analytics</strong> <strong>Survey</strong> <strong>2014</strong><br />
Leverage analytics,<br />
mitigate risks<br />
The following section is a collection of case studies across multiple<br />
industries where we have observed clients successfully deploying<br />
the right people, processes and technologies around FDA.<br />
Pharmaceutical company<br />
A leading global pharmaceutical company integrated FDA to assist in compliance<br />
monitoring between their sales representatives (REPs) and the health care professionals<br />
(HCPs) they interact with in certain high-risk countries. Whereas traditional FDA thinking<br />
would consider only one data source for analysis, this company incorporated big data<br />
thinking and integrated multiple structured and unstructured data sources with more<br />
sophisticated applications — in addition to spreadsheet and database applications.<br />
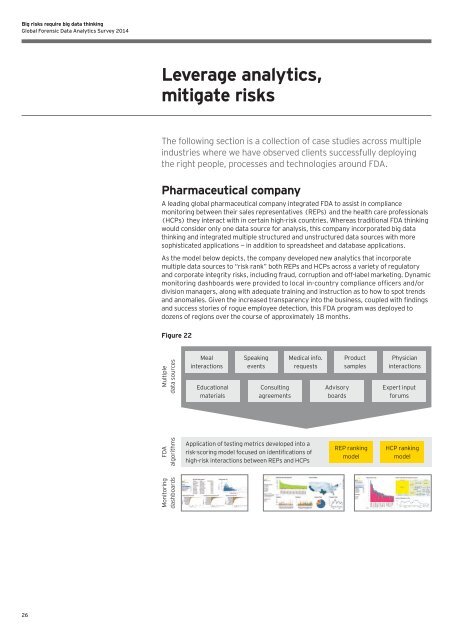
As the model below depicts, the company developed new analytics that incorporate<br />
multiple data sources to “risk rank” both REPs and HCPs across a variety of regulatory<br />
and corporate integrity risks, including fraud, corruption and off-label marketing. Dynamic<br />
monitoring dashboards were provided to local in-country compliance officers and/or<br />
division managers, along with adequate training and instruction as to how to spot trends<br />
and anomalies. Given the increased transparency into the business, coupled with findings<br />
and success stories of rogue employee detection, this FDA program was deployed to<br />
dozens of regions over the course of approximately 18 months.<br />
Figure 22<br />
Multiple<br />
data sources<br />
Meal<br />
interactions<br />
Educational<br />
materials<br />
Speaking<br />
events<br />
Consulting<br />
agreements<br />
Medical info.<br />
requests<br />
Advisory<br />
boards<br />
Product<br />
samples<br />
Physician<br />
interactions<br />
Expert input<br />
forums<br />
FDA<br />
algorithms<br />
Application of testing metrics developed into a<br />
risk-scoring model focused on identifications of<br />
high-risk interactions between REPs and HCPs<br />
REP ranking<br />
model<br />
HCP ranking<br />
model<br />
Monitoring<br />
dashboards<br />
26