for covalent compounds or ions
for covalent compounds or ions
for covalent compounds or ions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
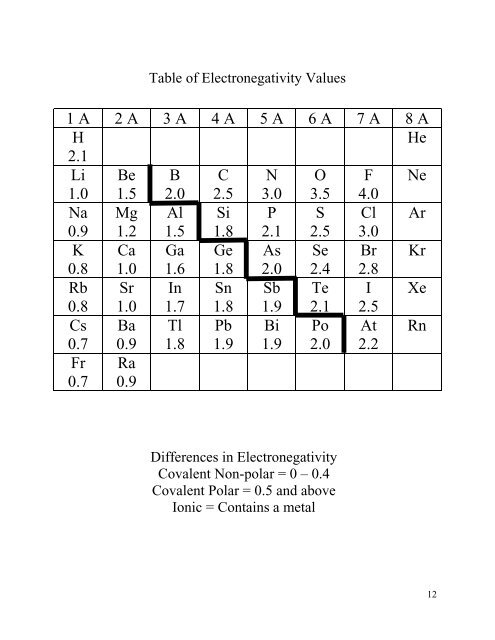
Table of Electronegativity Values<br />
1 A 2 A 3 A 4 A 5 A 6 A 7 A 8 A<br />
H<br />
He<br />
2.1<br />
Li Be B C N O F Ne<br />
1.0 1.5 2.0 2.5 3.0 3.5 4.0<br />
Na Mg Al Si P S Cl Ar<br />
0.9 1.2 1.5 1.8 2.1 2.5 3.0<br />
K Ca Ga Ge As Se Br Kr<br />
0.8 1.0 1.6 1.8 2.0 2.4 2.8<br />
Rb Sr In Sn Sb Te I Xe<br />
0.8 1.0 1.7 1.8 1.9 2.1 2.5<br />
Cs Ba Tl Pb Bi Po At Rn<br />
0.7 0.9 1.8 1.9 1.9 2.0 2.2<br />
Fr<br />
0.7<br />
Ra<br />
0.9<br />
Differences in Electronegativity<br />
Covalent Non-polar = 0 – 0.4<br />
Covalent Polar = 0.5 and above<br />
Ionic = Contains a metal<br />
12