ε-Fe2O3: An Advanced Nanomaterial Exhibiting Giant Coercive ...
ε-Fe2O3: An Advanced Nanomaterial Exhibiting Giant Coercive ...
ε-Fe2O3: An Advanced Nanomaterial Exhibiting Giant Coercive ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
______________________________________________________________________________<br />
6492 Chem. Mater., Vol. 22, No. 24, 2010 Tucek et al.<br />
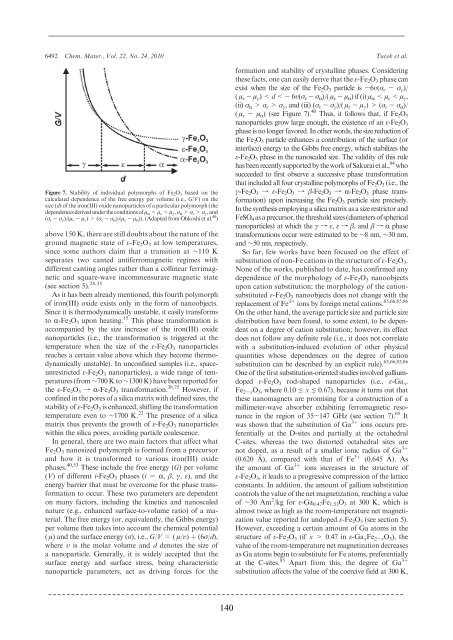
Figure 7. Stability of individual polymorphs of Fe 2 O 3 based on the<br />
calculated dependence of the free energy per volume (i.e., G/V) onthe<br />
size (d) of the iron(III) oxide nanoparticles of a particular polymorph (the<br />
dependences derived under the conditions of μ R σ ε >σ γ ,and<br />
(σ ε - σ γ )/(μ ε - μ γ )>(σ ε - σ R )/(μ ε - μ R )). (Adapted from Ohkoshi et al. 40 )<br />
above 150 K, there are still doubts about the nature of the<br />
ground magnetic state of ε-Fe 2 O 3 at low temperatures,<br />
since some authors claim that a transition at ∼110 K<br />
separates two canted antiferromagnetic regimes with<br />
different canting angles rather than a collinear ferrimagnetic<br />
and square-wave incommensurate magnetic state<br />
(see section 5). 28,35<br />
As it has been already mentioned, this fourth polymorph<br />
of iron(III) oxide exists only in the form of nanoobjects.<br />
Since it is thermodynamically unstable, it easily transforms<br />
to R-Fe 2 O 3 upon heating. 15 This phase transformation is<br />
accompanied by the size increase of the iron(III) oxide<br />
nanoparticles (i.e., the transformation is triggered at the<br />
temperature when the size of the ε-Fe 2 O 3 nanoparticles<br />
reaches a certain value above which they become thermodynamically<br />
unstable). In unconfined samples (i.e., spaceunrestricted<br />
ε-Fe 2 O 3 nanoparticles), a wide range of temperatures<br />
(from ∼700 K to ∼1300 K) have been reported for<br />
the ε-Fe 2 O 3 f R-Fe 2 O 3 transformation. 38,75 However, if<br />
confined in the pores of a silica matrix with defined sizes, the<br />
stability of ε-Fe 2 O 3 is enhanced, shifting the transformation<br />
temperature even to ∼1700 K. 22 The presence of a silica<br />
matrix thus prevents the growth of ε-Fe 2 O 3 nanoparticles<br />
within the silica pores, avoiding particle coalescence.<br />
In general, there are two main factors that affect what<br />
Fe 2 O 3 nanosized polymorph is formed from a precursor<br />
and how it is transformed to various iron(III) oxide<br />
phases. 40,53 These include the free energy (G) per volume<br />
(V) of different i-Fe 2 O 3 phases (i = R, β, γ, ε), and the<br />
energy barrier that must be overcome for the phase transformation<br />
to occur. These two parameters are dependent<br />
on many factors, including the kinetics and nanoscaled<br />
nature (e.g., enhanced surface-to-volume ratio) of a material.<br />
The free energy (or, equivalently, the Gibbs energy)<br />
per volume then takes into account the chemical potential<br />
( μ) and the surface energy (σ), i.e., G/V =(μ/υ) þ (6σ/d),<br />
where υ is the molar volume and d denotes the size of<br />
a nanoparticle. Generally, it is widely accepted that the<br />
surface energy and surface stress, being characteristic<br />
nanoparticle parameters, act as driving forces for the<br />
formation and stability of crystalline phases. Considering<br />
these facts, one can easily derive that the ε-Fe 2 O 3 phase can<br />
exist when the size of the Fe 2 O 3 particle is -6υ(σ ε - σ γ )/<br />
( μ ε - μ γ ) σ ε > σ γ ,and(iii)(σ ε - σ γ )/( μ ε - μ γ )>(σ ε - σ R )/<br />
( μ ε - μ R )(seeFigure7). 40 Thus, it follows that, if Fe 2 O 3<br />
nanoparticles grow large enough, the existence of an ε-Fe 2 O 3<br />
phase is no longer favored. In other words, the size reduction of<br />
the Fe 2 O 3 particle enhances a contribution of the surface (or<br />
interface) energy to the Gibbs free energy, which stabilizes the<br />
ε-Fe 2 O 3 phase in the nanoscaled size. The validity of this rule<br />
has been recently supported by the work of Sakurai et al., 84 who<br />
succeeded to first observe a successive phase transformation<br />
that included all four crystalline polymorphs of Fe 2 O 3 (i.e., the<br />
γ-Fe 2 O 3 f ε-Fe 2 O 3 f β-Fe 2 O 3 f R-Fe 2 O 3 phase transformation)<br />
upon increasing the Fe 2 O 3 particle size precisely.<br />
In the synthesis employing a silica matrix as a size restrictor and<br />
FeSO 4 as a precursor, the threshold sizes (diameters of spherical<br />
nanoparticles) at which the γ f ε, ε f β, andβ f R phase<br />
transformations occur were estimated to be ∼8nm,∼30 nm,<br />
and ∼50 nm, respectively.<br />
So far, few works have been focused on the effect of<br />
substitution of non-Fe cations in the structure of ε-Fe 2 O 3 .<br />
None of the works, published to date, has confirmed any<br />
dependence of the morphology of ε-Fe 2 O 3 nanoobjects<br />
upon cation substitution; the morphology of the cationsubstituted<br />
ε-Fe 2 O 3 nanoobjects does not change with the<br />
replacement of Fe 3þ ions by foreign metal cations. 65,66,85,86<br />
On the other hand, the average particle size and particle size<br />
distribution have been found, to some extent, to be dependent<br />
on a degree of cation substitution; however, its effect<br />
does not follow any definite rule (i.e., it does not correlate<br />
with a substitution-induced evolution of other physical<br />
quantities whose dependences on the degree of cation<br />
substitution can be described by an explicit rule). 65,66,85,86<br />
One of the first substitution-oriented studies involved galliumdoped<br />
ε-Fe 2 O 3 rod-shaped nanoparticles (i.e., ε-Ga x-<br />
Fe 2-x O 3 ,where0.10e x e 0.67), because it turns out that<br />
these nanomagnets are promising for a construction of a<br />
millimeter-wave absorber exhibiting ferromagnetic resonance<br />
in the region of 35-147 GHz (see section 7). 65 It<br />
was shown that the substitution of Ga 3þ ions occurs preferentially<br />
at the D-sites and partially at the octahedral<br />
C-sites, whereas the two distorted octahedral sites are<br />
not doped, as a result of a smaller ionic radius of Ga 3þ<br />
(0.620 A˚), compared with that of Fe 3þ (0.645 A˚). As<br />
theamountofGa 3þ ions increases in the structure of<br />
ε-Fe 2 O 3 , it leads to a progressive compression of the lattice<br />
constants. In addition, the amount of gallium substitution<br />
controls the value of the net magnetization, reaching a value<br />
of ∼30 Am 2 /kg for ε-Ga 0.47 Fe 1.53 O 3 at 300 K, which is<br />
almost twice as high as the room-temperature net magnetization<br />
value reported for undoped ε-Fe 2 O 3 (see section 5).<br />
However, exceeding a certain amount of Ga atoms in the<br />
structure of ε-Fe 2 O 3 (if x >0.47inε-Ga x Fe 2-x O 3 ), the<br />
value of the room-temperature net magnetization decreases<br />
as Ga atoms begin to substitute for Fe atoms, preferentially<br />
at the C-sites. 85 Apart from this, the degree of Ga 3þ<br />
substitution affects the value of the coercive field at 300 K,<br />
¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯<br />
¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯ ¯<br />
140