- Page 1 and 2:
THE ALCOHOL TEXTBOOK 4 TH EDITION A
- Page 3 and 4:
iv T.P. Lyons Nottingham University
- Page 5 and 6:
vi T.P. Lyons Yeast and management
- Page 7 and 8:
viii T.P. Lyons 28 Biorefineries: t
- Page 9 and 10:
x T.P. Lyons Corn Wheat Barley STAR
- Page 11 and 12:
Ethanol industry today
- Page 13 and 14:
2 T.P. Lyons nowhere near the forec
- Page 15 and 16:
4 T.P. Lyons Thousands of barrels/d
- Page 17 and 18:
6 T.P. Lyons While few people will
- Page 19 and 20:
Raw material handling and processin
- Page 21 and 22:
10 D.R. Kelsall and T.P. Lyons show
- Page 23 and 24:
12 D.R. Kelsall and T.P. Lyons Raw
- Page 25 and 26:
14 D.R. Kelsall and T.P. Lyons Figu
- Page 27 and 28:
16 D.R. Kelsall and T.P. Lyons Grai
- Page 29 and 30:
18 D.R. Kelsall and T.P. Lyons Grai
- Page 31 and 32:
20 D.R. Kelsall and T.P. Lyons % ac
- Page 33 and 34:
Enzymatic conversion of starch to f
- Page 35 and 36:
Enzymatic conversion of starch to f
- Page 37 and 38:
Enzymatic conversion of starch to f
- Page 39 and 40:
Enzymatic conversion of starch to f
- Page 41 and 42:
Enzymatic conversion of starch to f
- Page 43 and 44:
Grain handling: a critical aspect o
- Page 45 and 46:
Grain handling: a critical aspect o
- Page 47 and 48:
Grain handling: a critical aspect o
- Page 49 and 50:
Grain handling: a critical aspect o
- Page 51 and 52:
Lignocellulosics to ethanol 41 Chap
- Page 53 and 54:
Lignocellulosics to ethanol 43 and
- Page 55 and 56:
Lignocellulosics to ethanol 45 Tabl
- Page 57 and 58:
Lignocellulosics to ethanol 47 impo
- Page 59 and 60:
Lignocellulosics to ethanol 49 In B
- Page 61 and 62:
Lignocellulosics to ethanol 51 GLUC
- Page 63 and 64:
Lignocellulosics to ethanol 53 hydr
- Page 65 and 66:
Lignocellulosics to ethanol 55 from
- Page 67 and 68:
Lignocellulosics to ethanol 57 cere
- Page 69 and 70:
60 N.T.T. Vinh WORLD CASSAVA PRODUC
- Page 71 and 72:
62 N.T.T. Vinh COOKING Cooking is t
- Page 73 and 74:
64 N.T.T. Vinh Dougrell, L.M. 1982.
- Page 75 and 76:
66 J. O’Shea Table 2. Internation
- Page 77 and 78:
68 J. O’Shea As a consequence, th
- Page 79 and 80:
70 J. O’Shea lactose is able to a
- Page 81 and 82:
72 J. O’Shea Table 7. Alcohol pla
- Page 83 and 84:
Treatment and fermentation of molas
- Page 85 and 86:
Treatment and fermentation of molas
- Page 87 and 88:
Treatment and fermentation of molas
- Page 89 and 90:
Treatment and fermentation of molas
- Page 91 and 92:
Treatment and fermentation of molas
- Page 93 and 94:
Yeast and management of fermentatio
- Page 95 and 96:
CELLS 86 I. Russell of unique strai
- Page 97 and 98:
88 I. Russell Fibrillar layer Cell
- Page 99 and 100:
90 I. Russell Table 1. Approximate
- Page 101 and 102:
92 I. Russell Glucose Fructose Plas
- Page 103 and 104:
94 I. Russell resulting in the form
- Page 105 and 106:
96 I. Russell anaerobic conditions
- Page 107 and 108:
98 I. Russell For distilled beverag
- Page 109 and 110:
100 I. Russell 16 14 12 Superstart
- Page 111 and 112:
102 I. Russell since this is when e
- Page 113 and 114:
104 I. Russell Pyruvate NADH CO 2 A
- Page 115 and 116:
106 I. Russell biotin and many requ
- Page 117 and 118:
108 I. Russell effect on yeast cell
- Page 119 and 120:
110 I. Russell caproate (apple/anis
- Page 121 and 122:
112 I. Russell biosynthetic pathway
- Page 123 and 124:
114 I. Russell Table 7. FAN levels
- Page 125 and 126:
116 I. Russell Table 10. Classes of
- Page 127 and 128:
118 I. Russell Acknowledgments The
- Page 129 and 130:
Practical management of yeast: conv
- Page 131 and 132:
Practical management of yeast: conv
- Page 133 and 134:
Practical management of yeast: conv
- Page 135 and 136:
Practical management of yeast: conv
- Page 137 and 138:
Practical management of yeast: conv
- Page 139 and 140:
Practical management of yeast: conv
- Page 141 and 142:
Practical management of yeast: conv
- Page 143 and 144:
136 W.M. Ingledew growth seen in ba
- Page 145 and 146:
138 W.M. Ingledew fermentors and a
- Page 147 and 148:
140 W.M. Ingledew contaminated in t
- Page 149 and 150:
142 W.M. Ingledew %" Stress occurs
- Page 151 and 152:
Understanding near infrared spectro
- Page 153 and 154:
Understanding near infrared spectro
- Page 155 and 156:
Understanding near infrared spectro
- Page 157 and 158:
Understanding near infrared spectro
- Page 159 and 160:
Understanding near infrared spectro
- Page 161 and 162:
Understanding near infrared spectro
- Page 163 and 164:
Understanding near infrared spectro
- Page 165 and 166:
Understanding near infrared spectro
- Page 167 and 168:
Understanding near infrared spectro
- Page 169 and 170:
Understanding near infrared spectro
- Page 171 and 172:
Understanding near infrared spectro
- Page 173 and 174:
Understanding near infrared spectro
- Page 175 and 176:
Understanding near infrared spectro
- Page 177 and 178:
Biotechnological applications of no
- Page 179 and 180:
Biotechnological applications of no
- Page 181 and 182:
Biotechnological applications of no
- Page 183 and 184:
Biotechnological applications of no
- Page 185 and 186:
Biotechnological applications of no
- Page 187 and 188:
Biotechnological applications of no
- Page 189 and 190:
Biotechnological applications of no
- Page 191 and 192:
Biotechnological applications of no
- Page 193 and 194:
Biotechnological applications of no
- Page 195 and 196:
Biotechnological applications of no
- Page 197 and 198:
Biotechnological applications of no
- Page 199 and 200:
Production of Scotch and Irish whis
- Page 201 and 202:
Production of Scotch and Irish whis
- Page 203 and 204:
Production of Scotch and Irish whis
- Page 205 and 206:
Production of Scotch and Irish whis
- Page 207 and 208:
Production of Scotch and Irish whis
- Page 209 and 210:
Production of Scotch and Irish whis
- Page 211 and 212:
Production of Scotch and Irish whis
- Page 213 and 214:
Production of Scotch and Irish whis
- Page 215 and 216:
Production of Scotch and Irish whis
- Page 217 and 218:
Production of Scotch and Irish whis
- Page 219 and 220:
Production of Scotch and Irish whis
- Page 221 and 222:
Production of Scotch and Irish whis
- Page 223 and 224:
Production of Scotch and Irish whis
- Page 225 and 226:
Production of Scotch and Irish whis
- Page 227 and 228:
Production of Scotch and Irish whis
- Page 229 and 230:
Tequila production from agave 223 C
- Page 231 and 232:
Tequila production from agave 225 F
- Page 233 and 234:
Tequila production from agave 227 i
- Page 235 and 236:
Tequila production from agave 229 d
- Page 237 and 238:
Tequila production from agave 231 d
- Page 239 and 240:
Tequila production from agave 233 a
- Page 241 and 242:
Tequila production from agave 235 5
- Page 243 and 244:
Tequila production from agave 237 8
- Page 245 and 246:
Tequila production from agave 239 D
- Page 247 and 248:
Tequila production from agave 241 s
- Page 249 and 250:
Tequila production from agave 243 1
- Page 251 and 252:
Tequila production from agave 245 t
- Page 253 and 254:
248 R. Piggot alcohol is made from
- Page 255 and 256:
250 R. Piggot commercial distillers
- Page 257 and 258:
252 R. Piggot 6 5 4 Scotch Dark Rum
- Page 259 and 260:
From pot stills to continuous still
- Page 261 and 262:
From pot stills to continuous still
- Page 263 and 264:
From pot stills to continuous still
- Page 265 and 266:
From pot stills to continuous still
- Page 267 and 268:
From pot stills to continuous still
- Page 269 and 270:
From pot stills to continuous still
- Page 271 and 272:
From liqueurs to ‘malternatives
- Page 273 and 274: From liqueurs to ‘malternatives
- Page 275 and 276: From liqueurs to ‘malternatives
- Page 277 and 278: From liqueurs to ‘malternatives
- Page 279 and 280: 276 R. Ralph History of North Ameri
- Page 281 and 282: 278 R. Ralph DISTILLATION’S NEW E
- Page 283 and 284: 280 R. Ralph Cyclone Grain cleaner
- Page 285 and 286: 282 R. Ralph and let the fermentors
- Page 287 and 288: 284 R. Ralph water/grain mixture on
- Page 289 and 290: Contamination and hygiene
- Page 291 and 292: 288 N.V. Narendranath Diagnostic ch
- Page 293 and 294: 290 N.V. Narendranath this to pento
- Page 295 and 296: 292 N.V. Narendranath ACETIC ACID (
- Page 297 and 298: 294 N.V. Narendranath Figure 5. A t
- Page 299 and 300: 296 N.V. Narendranath 3. Control th
- Page 301 and 302: 298 N.V. Narendranath during alcoho
- Page 303 and 304: 300 J. Larson and J. Power For any
- Page 305 and 306: 302 J. Larson and J. Power Money %
- Page 307 and 308: 304 J. Larson and J. Power warmer o
- Page 309 and 310: 306 J. Larson and J. Power a chelat
- Page 311 and 312: 308 J. Larson and J. Power It is a
- Page 313 and 314: 310 J. Larson and J. Power Plate he
- Page 315 and 316: 312 J. Larson and J. Power 2. Made
- Page 317 and 318: 314 J. Larson and J. Power Temperat
- Page 319 and 320: 316 J. Larson and J. Power • Dead
- Page 321 and 322: 318 J. Larson and J. Power specifie
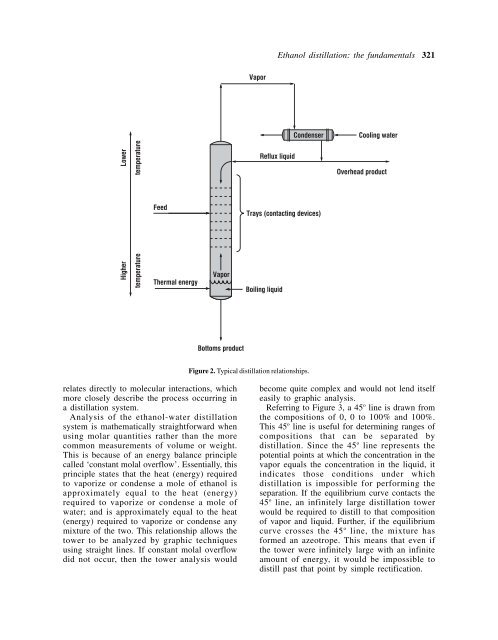
- Page 323: Ethanol distillation: the fundament
- Page 327 and 328: Ethanol distillation: the fundament
- Page 329 and 330: Ethanol distillation: the fundament
- Page 331 and 332: Ethanol distillation: the fundament
- Page 333 and 334: Ethanol distillation: the fundament
- Page 335 and 336: Ethanol distillation: the fundament
- Page 337 and 338: Ethanol distillation: the fundament
- Page 339 and 340: Ethanol distillation: the fundament
- Page 341 and 342: Development and operation of the mo
- Page 343 and 344: Development and operation of the mo
- Page 345 and 346: Development and operation of the mo
- Page 347 and 348: Water reuse in fuel alcohol plants:
- Page 349 and 350: Water reuse in fuel alcohol plants:
- Page 351 and 352: Water reuse in fuel alcohol plants:
- Page 353 and 354: Water reuse in fuel alcohol plants:
- Page 355 and 356: Water reuse in fuel alcohol plants:
- Page 357 and 358: Water reuse in fuel alcohol plants:
- Page 359 and 360: Understanding energy use and energy
- Page 361 and 362: Understanding energy use and energy
- Page 363 and 364: Understanding energy use and energy
- Page 365 and 366: Understanding energy use and energy
- Page 367 and 368: Dryhouse design: focusing on reliab
- Page 369 and 370: Dryhouse design: focusing on reliab
- Page 371 and 372: Dryhouse design: focusing on reliab
- Page 373 and 374: Dryhouse design: focusing on reliab
- Page 375 and 376:
Dryhouse design: focusing on reliab
- Page 377 and 378:
Dryhouse design: focusing on reliab
- Page 379 and 380:
Dryhouse design: focusing on reliab
- Page 381 and 382:
378 K.A. Jacques There is a growing
- Page 383 and 384:
380 K.A. Jacques O Fe H O P O O O O
- Page 385 and 386:
382 K.A. Jacques converting it to m
- Page 387 and 388:
384 K.A. Jacques spread manure thre
- Page 389 and 390:
Biorefineries: the versatile fermen
- Page 391 and 392:
Biorefineries: the versatile fermen
- Page 393 and 394:
Biorefineries: the versatile fermen
- Page 395 and 396:
Biorefineries: the versatile fermen
- Page 397 and 398:
Biorefineries: the versatile fermen
- Page 399 and 400:
Biorefineries: the versatile fermen
- Page 401 and 402:
400 The Alcohol Alphabet Acidity A
- Page 403 and 404:
402 The Alcohol Alphabet Arabinose
- Page 405 and 406:
404 The Alcohol Alphabet Binary aze
- Page 407 and 408:
406 The Alcohol Alphabet grown in t
- Page 409 and 410:
408 The Alcohol Alphabet Concentrat
- Page 411 and 412:
410 The Alcohol Alphabet phase is s
- Page 413 and 414:
412 The Alcohol Alphabet Distillery
- Page 415 and 416:
414 The Alcohol Alphabet of ethanol
- Page 417 and 418:
416 The Alcohol Alphabet in suspens
- Page 419 and 420:
418 The Alcohol Alphabet Glucose is
- Page 421 and 422:
420 The Alcohol Alphabet Hydrometer
- Page 423 and 424:
422 The Alcohol Alphabet L Lactase
- Page 425 and 426:
424 The Alcohol Alphabet and saccha
- Page 427 and 428:
426 The Alcohol Alphabet sieve mate
- Page 429 and 430:
428 The Alcohol Alphabet knock and
- Page 431 and 432:
430 The Alcohol Alphabet Plate (or
- Page 433 and 434:
432 The Alcohol Alphabet the refrac
- Page 435 and 436:
434 The Alcohol Alphabet Solute One
- Page 437 and 438:
436 The Alcohol Alphabet TBA Abbrev
- Page 439 and 440:
438 The Alcohol Alphabet Vaporizati
- Page 441 and 442:
Index 441 Index Acetobacter (see Ba
- Page 443 and 444:
Index 443 Thermocompressors 265, 33
- Page 445 and 446:
Index 445 Quaternary ammonium compo
- Page 448:
THE ALCOHOL TEXTBOOK 4 TH EDITION A