reference medicinal product - TOPRA
reference medicinal product - TOPRA
reference medicinal product - TOPRA
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
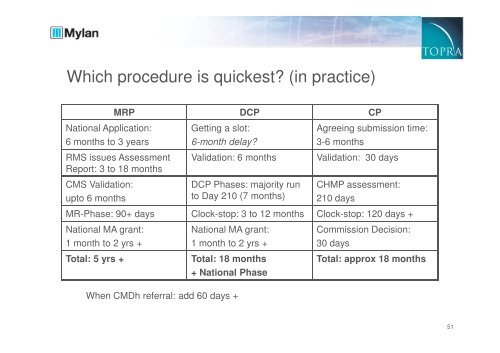
Which procedure is quickest? (in practice)<br />
MRP DCP CP<br />
National Application:<br />
6 months to 3 years<br />
RMS issues Assessment<br />
Report: 3 to 18 months<br />
CMS Validation:<br />
upto 6 months<br />
Getting a slot:<br />
6-month delay?<br />
Validation: 6 months<br />
DCP Phases: majority run<br />
to Day 210 (7 months)<br />
Agreeing submission time:<br />
3-6 months<br />
Validation: 30 days<br />
CHMP assessment:<br />
210 days<br />
MR-Phase: 90+ days Clock-stop: 3 to 12 months Clock-stop: 120 days +<br />
National MA grant:<br />
1 month to 2 yrs +<br />
Total: 5 yrs +<br />
National MA grant:<br />
1 month to 2 yrs +<br />
Total: 18 months<br />
+ National Phase<br />
Commission Decision:<br />
30 days<br />
Total: approx 18 months<br />
When CMDh referral: add 60 days +<br />
51