Download pdf file - IPRAS
Download pdf file - IPRAS
Download pdf file - IPRAS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
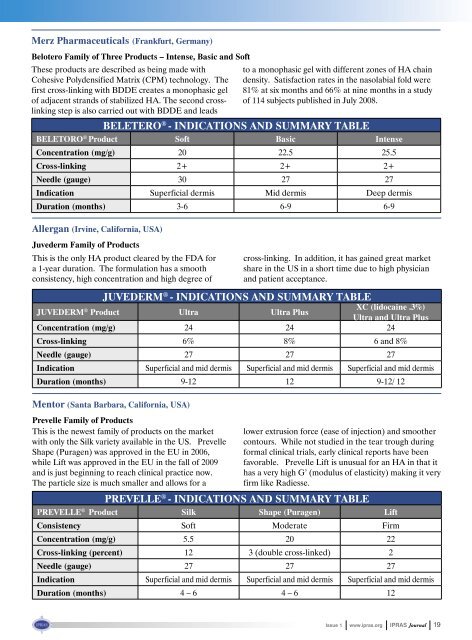
Merz Pharmaceuticals (Frankfurt, Germany)<br />
Belotero Family of Three Products – Intense, Basic and Soft<br />
These products are described as being made with<br />
Cohesive Polydensified Matrix (CPM) technology. The<br />
first cross-linking with BDDE creates a monophasic gel<br />
of adjacent strands of stabilized HA. The second crosslinking<br />
step is also carried out with BDDE and leads<br />
to a monophasic gel with different zones of HA chain<br />
density. Satisfaction rates in the nasolabial fold were<br />
81% at six months and 66% at nine months in a study<br />
of 114 subjects published in July 2008.<br />
BELETERO ® - INDICATIONS AND SUMMARY TABLE<br />
BELETORO ® Product Soft Basic Intense<br />
Concentration (mg/g) 20 22.5 25.5<br />
Cross-linking 2+ 2+ 2+<br />
Needle (gauge) 30 27 27<br />
Indication Superficial dermis Mid dermis Deep dermis<br />
Duration (months) 3-6 6-9 6-9<br />
Allergan (Irvine, California, USA)<br />
Juvederm Family of Products<br />
This is the only HA product cleared by the FDA for<br />
a 1-year duration. The formulation has a smooth<br />
consistency, high concentration and high degree of<br />
cross-linking. In addition, it has gained great market<br />
share in the US in a short time due to high physician<br />
and patient acceptance.<br />
JUVEDERM ® - INDICATIONS AND SUMMARY TABLE<br />
JUVEDERM ® Product Ultra Ultra Plus<br />
XC (lidocaine .3%)<br />
Ultra and Ultra Plus<br />
Concentration (mg/g) 24 24 24<br />
Cross-linking 6% 8% 6 and 8%<br />
Needle (gauge) 27 27 27<br />
Indication Superficial and mid dermis Superficial and mid dermis Superficial and mid dermis<br />
Duration (months) 9-12 12 9-12/ 12<br />
Mentor (Santa Barbara, California, USA)<br />
Prevelle Family of Products<br />
This is the newest family of products on the market<br />
with only the Silk variety available in the US. Prevelle<br />
Shape (Puragen) was approved in the EU in 2006,<br />
while Lift was approved in the EU in the fall of 2009<br />
and is just beginning to reach clinical practice now.<br />
The particle size is much smaller and allows for a<br />
lower extrusion force (ease of injection) and smoother<br />
contours. While not studied in the tear trough during<br />
formal clinical trials, early clinical reports have been<br />
favorable. Prevelle Lift is unusual for an HA in that it<br />
has a very high G’ (modulus of elasticity) making it very<br />
firm like Radiesse.<br />
PREVELLE ® - INDICATIONS AND SUMMARY TABLE<br />
PREVELLE ® Product Silk Shape (Puragen) Lift<br />
Consistency Soft Moderate Firm<br />
Concentration (mg/g) 5.5 20 22<br />
Cross-linking (percent) 12 3 (double cross-linked) 2<br />
Needle (gauge) 27 27 27<br />
Indication Superficial and mid dermis Superficial and mid dermis Superficial and mid dermis<br />
Duration (months) 4 – 6 4 – 6 12<br />
Issue 1 www.ipras.org <strong>IPRAS</strong> Journal 19