Simultaneous Determination of Chelating Agents
Simultaneous Determination of Chelating Agents
Simultaneous Determination of Chelating Agents
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Dodi and Bouscarel<br />
increased at 1.1 mL/min, the pressure reached values <strong>of</strong><br />
approximately 17.6 MPa (2550 psi).<br />
As we can see in Figure 3, the elution order is different from<br />
the one observed for ion-suppression chromatography. In fact,<br />
the retention time seems to be correlated with the number <strong>of</strong><br />
carboxylic functions present in the compound; the more the<br />
chelating agent has carboxylic functions, the more the ferric<br />
complex is retained on the stationary phase.<br />
We must note that CDTA constitutes an exception because<br />
this molecule exhibits a hydrophobic structure (C 6 cycle)<br />
(Figure 1) and thus, strong hydrophobic interactions occur<br />
between CDTA and the alkyl groups <strong>of</strong> the stationary phase.<br />
Linearity was observed up to 500 µg/L. The least-squares<br />
equations and the correlation coefficients for each compound<br />
are presented in Table 3.<br />
<strong>Chelating</strong> agents must be taken into<br />
account in the management <strong>of</strong> (nuclear)<br />
waste because they can induce watersoluble<br />
complexes with most heavy metals.<br />
copper, cobalt and lead. We prepared aqueous solutions with a<br />
10 3 ratio metal/chelating agent.<br />
In this instance, a complex was formed between the metal<br />
and the chelating agent but a metal excess remained in the<br />
solution. The samples were investigated by ion pair<br />
chromatography and the results are reported in Figure 4. We<br />
may observe that cobalt modified the concentration <strong>of</strong> NTA<br />
and CDTA, copper modified the concentration <strong>of</strong> CDTA and<br />
DTPA and lead did not alter any concentration.<br />
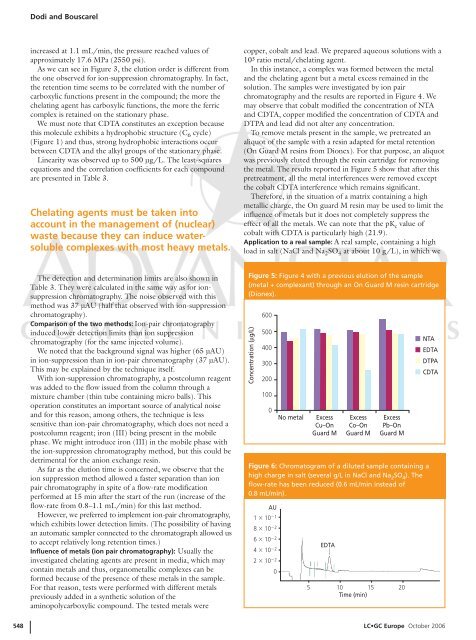
To remove metals present in the sample, we pretreated an<br />
aliquot <strong>of</strong> the sample with a resin adapted for metal retention<br />
(On Guard M resins from Dionex). For that purpose, an aliquot<br />
was previously eluted through the resin cartridge for removing<br />
the metal. The results reported in Figure 5 show that after this<br />
pretreatment, all the metal interferences were removed except<br />
the cobalt CDTA interference which remains significant.<br />
Therefore, in the situation <strong>of</strong> a matrix containing a high<br />
metallic charge, the On guard M resin may be used to limit the<br />
influence <strong>of</strong> metals but it does not completely suppress the<br />
effect <strong>of</strong> all the metals. We can note that the pK c value <strong>of</strong><br />
cobalt with CDTA is particularly high (21.9).<br />
Application to a real sample: A real sample, containing a high<br />
load in salt (NaCl and Na 2 SO 4 at about 10 g/L), in which we<br />
The detection and determination limits are also shown in<br />
Table 3. They were calculated in the same way as for ionsuppression<br />
chromatography. The noise observed with this<br />
method was 37 µAU (half that observed with ion-suppression<br />
chromatography).<br />
Comparison <strong>of</strong> the two methods: Ion-pair chromatography<br />
induced lower detection limits than ion suppression<br />
chromatography (for the same injected volume).<br />
We noted that the background signal was higher (65 µAU)<br />
in ion-suppression than in ion-pair chromatography (37 µAU).<br />
This may be explained by the technique itself.<br />
With ion-suppression chromatography, a postcolumn reagent<br />
was added to the flow issued from the column through a<br />
mixture chamber (thin tube containing micro balls). This<br />
operation constitutes an important source <strong>of</strong> analytical noise<br />
and for this reason, among others, the technique is less<br />
sensitive than ion-pair chromatography, which does not need a<br />
postcolumn reagent; iron (III) being present in the mobile<br />
phase. We might introduce iron (III) in the mobile phase with<br />
the ion-suppression chromatography method, but this could be<br />
detrimental for the anion exchange resin.<br />
As far as the elution time is concerned, we observe that the<br />
ion suppression method allowed a faster separation than ion<br />
pair chromatography in spite <strong>of</strong> a flow-rate modification<br />
performed at 15 min after the start <strong>of</strong> the run (increase <strong>of</strong> the<br />
flow-rate from 0.8–1.1 mL/min) for this last method.<br />
However, we preferred to implement ion-pair chromatography,<br />
which exhibits lower detection limits. (The possibility <strong>of</strong> having<br />
an automatic sampler connected to the chromatograph allowed us<br />
to accept relatively long retention times.)<br />
Influence <strong>of</strong> metals (ion pair chromatography): Usually the<br />
investigated chelating agents are present in media, which may<br />
contain metals and thus, organometallic complexes can be<br />
formed because <strong>of</strong> the presence <strong>of</strong> these metals in the sample.<br />
For that reason, tests were performed with different metals<br />
previously added in a synthetic solution <strong>of</strong> the<br />
aminopolycarboxylic compound. The tested metals were<br />
Figure 5: Figure 4 with a previous elution <strong>of</strong> the sample<br />
(metal + complexant) through an On Guard M resin cartridge<br />
(Dionex).<br />
Concentration (µg/L)<br />
600<br />
500<br />
400<br />
300<br />
200<br />
100<br />
0<br />
Figure 6: Chromatogram <strong>of</strong> a diluted sample containing a<br />
high charge in salt (several g/L in NaCl and Na 2 SO 4 ). The<br />
flow-rate has been reduced (0.6 mL/min instead <strong>of</strong><br />
0.8 mL/min).<br />
AU<br />
No metal<br />
Excess<br />
Cu–On<br />
Guard M<br />
1 10 1<br />
8 10 2<br />
6 10 2<br />
EDTA<br />
4 10 2<br />
2 10 2 0<br />
Excess<br />
Co–On<br />
Guard M<br />
Excess<br />
Pb–On<br />
Guard M<br />
5 10 15 20<br />
Time (min)<br />
NTA<br />
EDTA<br />
DTPA<br />
CDTA<br />
548<br />
LC•GC Europe October 2006