How is the quality of vaccines assured by the ... - The INCLEN Trust
How is the quality of vaccines assured by the ... - The INCLEN Trust
How is the quality of vaccines assured by the ... - The INCLEN Trust
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
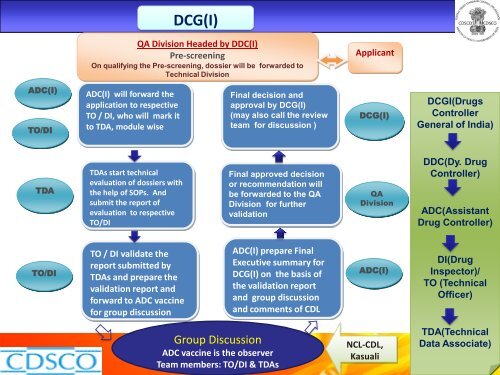
DCG(I)<br />
QA Div<strong>is</strong>ion Headed <strong>by</strong> DDC(I)<br />
Pre-screening<br />
On qualifying <strong>the</strong> Pre-screening, dossier will be forwarded to<br />
Technical Div<strong>is</strong>ion<br />
Applicant<br />
ADC(I)<br />
TO/DI<br />
ADC(I) will forward <strong>the</strong><br />
application to respective<br />
TO / DI, who will mark it<br />
to TDA, module w<strong>is</strong>e<br />
Final dec<strong>is</strong>ion and<br />
approval <strong>by</strong> DCG(I)<br />
(may also call <strong>the</strong> review<br />
team for d<strong>is</strong>cussion )<br />
DCG(I)<br />
DCGI(Drugs<br />
Controller<br />
General <strong>of</strong> India)<br />
TDA<br />
TDAs start technical<br />
evaluation <strong>of</strong> dossiers with<br />
<strong>the</strong> help <strong>of</strong> SOPs. And<br />
submit <strong>the</strong> report <strong>of</strong><br />
evaluation to respective<br />
TO/DI<br />
Final approved dec<strong>is</strong>ion<br />
or recommendation will<br />
be forwarded to <strong>the</strong> QA<br />
Div<strong>is</strong>ion for fur<strong>the</strong>r<br />
validation<br />
QA<br />
Div<strong>is</strong>ion<br />
DDC(Dy. Drug<br />
Controller)<br />
ADC(Ass<strong>is</strong>tant<br />
Drug Controller)<br />
TO/DI<br />
TO / DI validate <strong>the</strong><br />
report submitted <strong>by</strong><br />
TDAs and prepare <strong>the</strong><br />
validation report and<br />
forward to ADC vaccine<br />
for group d<strong>is</strong>cussion<br />
ADC(I) prepare Final<br />
Executive summary for<br />
DCG(I) on <strong>the</strong> bas<strong>is</strong> <strong>of</strong><br />
<strong>the</strong> validation report<br />
and group d<strong>is</strong>cussion<br />
and comments <strong>of</strong> CDL<br />
ADC(I)<br />
DI(Drug<br />
Inspector)/<br />
TO (Technical<br />
Officer)<br />
Group D<strong>is</strong>cussion<br />
ADC vaccine <strong>is</strong> <strong>the</strong> observer<br />
Team members: TO/DI & TDAs<br />
NCL-CDL,<br />
Kasuali<br />
TDA(Technical<br />
Data Associate)