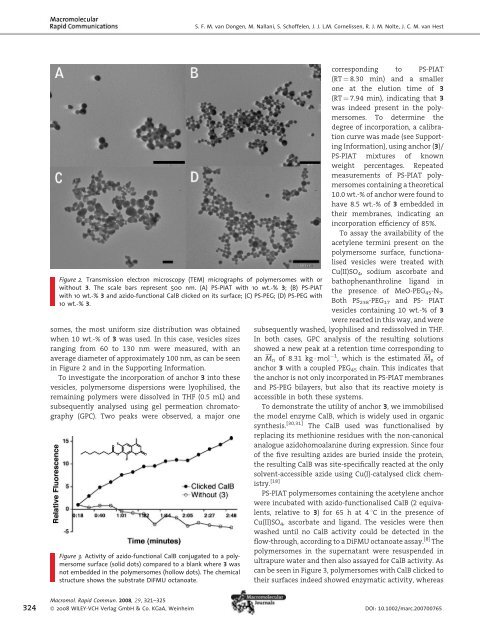
S. F. M. van Dongen, M. Nallani, S. Sch<strong>of</strong>felen, J. J. L.M. Cornelissen, R. J. M. Nolte, J. C. M. van Hest Figure 2. Transmission electron microscopy (TEM) micrographs <strong>of</strong> polymersomes with or without 3. The scale bars represent 500 nm. (A) PS-PIAT with 10 wt.-% 3; (B) PS-PIAT with 10 wt.-% 3 and azido-functional CalB clicked on its surface; (C) PS-PEG; (D) PS-PEG with 10 wt.-% 3. somes, the most uni<strong>for</strong>m size distribution was obtained when 10 wt.-% <strong>of</strong> 3 was used. In this case, vesicles sizes ranging from 60 to 130 nm were measured, with an average diameter <strong>of</strong> approximately 100 nm, as can be seen in Figure 2 and in the Supporting In<strong>for</strong>mation. To investigate the incorporation <strong>of</strong> anchor 3 into these vesicles, polymersome dispersions were lyophilised, the remaining polymers were dissolved in THF (0.5 mL) and subsequently analysed using gel permeation chromatography (GPC). Two peaks were observed, a major one Figure 3. Activity <strong>of</strong> azido-functional CalB conjugated to a polymersome surface (solid dots) compared to a blank where 3 was not embedded in the polymersomes (hollow dots). The chemical structure shows the substrate DiFMU octanoate. corresponding to PS-PIAT (RT ¼ 8.30 min) and a smaller one at the elution time <strong>of</strong> 3 (RT ¼ 7.94 min), indicating that 3 was indeed present in the polymersomes. To determine the degree <strong>of</strong> incorporation, a calibration curve was made (see Supporting In<strong>for</strong>mation), using anchor (3)/ PS-PIAT mixtures <strong>of</strong> known weight percentages. Repeated measurements <strong>of</strong> PS-PIAT polymersomes containing a theoretical 10.0 wt.-% <strong>of</strong> anchor were found to have 8.5 wt.-% <strong>of</strong> 3 embedded in their membranes, indicating an incorporation efficiency <strong>of</strong> 85%. To assay the availability <strong>of</strong> the acetylene termini present on the polymersome surface, functionalised vesicles were treated with Cu(II)SO 4 , sodium ascorbate and bathophenanthroline ligand in the presence <strong>of</strong> MeO-PEG 45 -N 3 . Both PS 238 -PEG 17 and PS- PIAT vesicles containing 10 wt.-% <strong>of</strong> 3 were reacted in this way, and were subsequently washed, lyophilised and redissolved in THF. In both cases, GPC analysis <strong>of</strong> the resulting solutions showed a new peak at a retention time corresponding to an M n <strong>of</strong> 8.31 kg mol 1 , which is the estimated M n <strong>of</strong> anchor 3 with a coupled PEG 45 chain. This indicates that the anchor is not only incorporated in PS-PIAT membranes and PS-PEG bilayers, but also that its reactive moiety is accessible in both these systems. To demonstrate the utility <strong>of</strong> anchor 3, we immobilised the model enzyme CalB, which is widely used in organic synthesis. [30,31] The CalB used was functionalised by replacing its methionine residues with the non-canonical analogue azidohomoalanine during expression. Since four <strong>of</strong> the five resulting azides are buried inside the protein, the resulting CalB was site-specifically reacted at the only solvent-accessible azide using Cu(I)-catalysed click chemistry. [18] PS-PIAT polymersomes containing the acetylene anchor were incubated with azido-functionalised CalB (2 equivalents, relative to 3) <strong>for</strong> 65 h at 4 8C in the presence <strong>of</strong> Cu(II)SO 4 , ascorbate and ligand. The vesicles were then washed until no CalB activity could be detected in the flow-through, according to a DiFMU octanoate assay. [8] The polymersomes in the supernatant were resuspended in ultrapure water and then also assayed <strong>for</strong> CalB activity. As can be seen in Figure 3, polymersomes with CalB clicked to their surfaces indeed showed enzymatic activity, whereas 324 Macromol. Rapid Commun. 2008, 29, 321–325 ß 2008 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim DOI: 10.1002/marc.200700765
A <strong>Block</strong> <strong>Copolymer</strong> <strong>for</strong> <strong>Functionalisation</strong> <strong>of</strong> <strong>Polymer</strong>some Surfaces control experiments, where either the anchor or the Cu(I)-catalyst were omitted, did not. The vesicle morphology did not change significantly upon enzyme conjugation, as can be seen in Figure 2. To our knowledge, this is the first example <strong>of</strong> the immobilisation <strong>of</strong> a catalytically active enzyme on the surface <strong>of</strong> a polymersome. As was demonstrated by our washing procedure, these decorated polymersomes can be fully removed from an aqueous sample, which enables catalyst recovery. Furthermore, anchor 3 establishes itself as a convenient way to introduce functionality on otherwise unmodifiable surfaces. This trait does not limit itself to enzyme immobilisation: it may find applications in polymersome labelling using dyes, biological signalling molecules or even immobilisation <strong>of</strong> polymersomes on larger surfaces. Conclusion We synthesised a PS-PEG block copolymer which terminates with an acetylene moiety at its hydrophilic end. This so-called anchor can coaggregate with different types <strong>of</strong> other block copolymers (i.e. PS-PIAT or PS-PEG) during the <strong>for</strong>mation <strong>of</strong> polymersomes, as proven by GPC analysis <strong>of</strong> redissolved vesicles. The acetylene group thus presented at the surface <strong>of</strong> the polymersome is an accessible functional handle <strong>for</strong> (bio-)conjugation <strong>of</strong> azido-compounds. To prove this, the anchor was incorporated in porous PS-PIAT polymersomes, the surfaces <strong>of</strong> which cannot normally be functionalised. Conjugation <strong>of</strong> azido-CalB to the resulting vesicles produced biohybrid polymersomes that indeed showed enzymatic activity, as shown by DiFMU assays. This anchor introduces the polymersome surface as a locale <strong>for</strong> enzyme immobilisation, complementary to the polymersome lumen and bilayer. [8] Acknowledgements: The authors acknowledge the Netherlands National Research School Combination Catalysis (NRSC-C) <strong>for</strong> financial support. We thank Joost Opsteen <strong>for</strong> the synthesis <strong>of</strong> azido-PEG. Received: October 28, 2007; Accepted: November 19, 2007; DOI: 10.1002/marc.200700765 Keywords: diblock copolymers; enzymes; polymersomes; sel<strong>for</strong>ganisation; surface functionalization [1] D. E. Discher, A. Eisenberg, Science 2002, 297, 967. [2] L. B. Luo, A. Eisenberg, Langmuir 2001, 17, 6804. [3] B. M. Discher, H. Bermudez, D. A. Hammer, D. E. Discher, Y. Y. Won, F. S. Bates, J. Phys. Chem. B 2002, 106, 2848. [4] F. H. Meng, C. Hiemstra, G. H. M. Engbers, J. Feijen, Macromolecules 2003, 36, 3004. [5] F. Ahmed, R. I. Pakunlu, A. Brannan, F. Bates, T. Minko, D. E. Discher, J. Controlled Release 2006, 116, 150. [6] N. A. Christian, M. C. Milone, S. S. Ranka, G. Z. Li, P. R. Frail, K. P. Davis, F. S. Bates, M. J. Therien, P. P. Ghoroghchian, C. H. June, D. A. Hammer, Bioconjug. Chem. 2007, 18, 31. [7] D. M. Vriezema, M. C. Aragones, J. A. A. W. Elemans, J. J. L. M. Cornelissen, A. E. Rowan, R. J. M. Nolte, Chem. Rev. 2005, 105, 1445. [8] D. M. Vriezema, P. M. L. Garcia, N. Sancho Oltra, N. S. Hatzakis, S. M. Kuiper, R. J. M. Nolte, A. E. Rowan, J. C. M. van Hest, Angew. Chem., Int. Ed. 2007, 46, 7378. [9] D. M. Vriezema, J. Hoogboom, K. Velonia, K. Takazawa, P. C. M. Christianen, J. C. Maan, A. E. Rowan, R. J. M. Nolte, Angew. Chem., Int. Ed. 2003, 42, 772. [10] J. J. Lin, P. Ghoroghchian, Y. Zhang, D. A. Hammer, Langmuir 2006, 22, 3975. [11] P. Broz, S. M. Benito, C. Saw, P. Burger, H. Heider, M. Pfisterer, S. Marsch, W. Meier, P. Hunziker, J. Controlled Release 2005, 102, 475. [12] D. M. Vriezema, A. Kros, R. de Gelder, J. J. L. M. Cornelissen, A. E. Rowan, R. J. M. Nolte, Macromolecules 2004, 37, 4736. [13] I. C. Reynhout, J. J. L. M. Cornelissen, R. J. M. Nolte, J. Am. Chem. Soc. 2007, 129, 2327. [14] J. A. Opsteen, R. P. Brinkhuis, R. L. M. Teeuwen, D. W. P. M. Löwik, J. C. M. van Hest, Chem. Commun. 2007, 3136. [15] S. W. A. Reulen, W. W. T. Brusselaars, S. Langereis, W. J. M. Mulder, M. Breurken, M. Merkx, Bioconjug. Chem. 2007, 18, 590. [16] S. Cavalli, A. R. Tipton, M. Overhand, A. Kros, Chem. Commun. 2006, 3193. [17] B. Li, A. L. Martin, E. R. Gillies, Chem. Commun. 2007, 5217. [18] S. Sch<strong>of</strong>felen, M. H. L. Lambermon, J. C. M. van Hest, submitted. [19] K. Yu, A. Eisenberg, Macromolecules 1996, 29, 6359. [20] J. A. Opsteen, J. C. M. van Hest, Chem. Commun. 2005, 57. [21] R. Huisgen, Angew. Chem., Int. Ed. 1963, 75, 604. [22] V. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem., Int. Ed. 2002, 41, 2596. [23] W. H. Binder, R. Sachsenh<strong>of</strong>er, Macromol. Rapid Commun. 2007, 28, 15. [24] J. F. Lutz, Angew. Chem., Int. Ed. 2007, 46, 1018. [25] Q. Wang, T. R. Chan, R. Hilgraf, V. V. Fokin, K. B. Sharpless, M. G. Finn, J. Am. Chem. Soc. 2003, 125, 3192. [26] A. J. T. Dirks, S. S. van Berkel, N. S. Hatzakis, J. A. Opsteen, F. L. van Delft, J. J. L. M. Cornelissen, A. E. Rowan, J. C. M. van Hest, F. P. J. T. Rutjes, R. J. M. Nolte, Chem. Commun. 2005, 4172. [27] J. S. Wang, K. Matyjaszewski, Macromolecules 1995, 28, 7901. [28] J. F. Lutz, H. G. Borner, K. Weichenhan, Macromolecules 2006, 39, 6376. [29] K. Matyjaszewski, Y. Nakagawa, S. G. Gaynor, Macromol. Rapid Commun. 1997, 18, 1057. [30] V. Gotor-Fernandez, E. Busto, V. Gotor, Adv. Synth. Catal. 2006, 348, 797. [31] J. Peeters, A. R. A. Palmans, M. Veld, F. Scheijen, A. Heise, E. W. Meijer, Biomacromolecules 2004, 5, 1862. Macromol. Rapid Commun. 2008, 29, 321–325 ß 2008 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.mrc-journal.de 325