Electrochemistry
Electrochemistry
Electrochemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
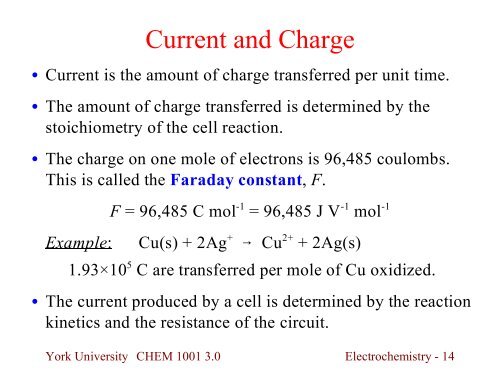
Current and Charge<br />
Current is the amount of charge transferred per unit time.<br />
The amount of charge transferred is determined by the<br />
stoichiometry of the cell reaction.<br />
The charge on one mole of electrons is 96,485 coulombs.<br />
This is called the Faraday constant, F.<br />
F = 96,485 C mol -1 = 96,485 J V -1 mol -1<br />
Example: Cu(s) + 2Ag + Cu 2+ + 2Ag(s)<br />
1.93×10 5 C are transferred per mole of Cu oxidized.<br />
The current produced by a cell is determined by the reaction<br />
kinetics and the resistance of the circuit.<br />
York University CHEM 1001 3.0 <strong>Electrochemistry</strong> - 14