Soil Science - Sameti.org
Soil Science - Sameti.org
Soil Science - Sameti.org
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Soil</strong> <strong>Science</strong><br />
II.<br />
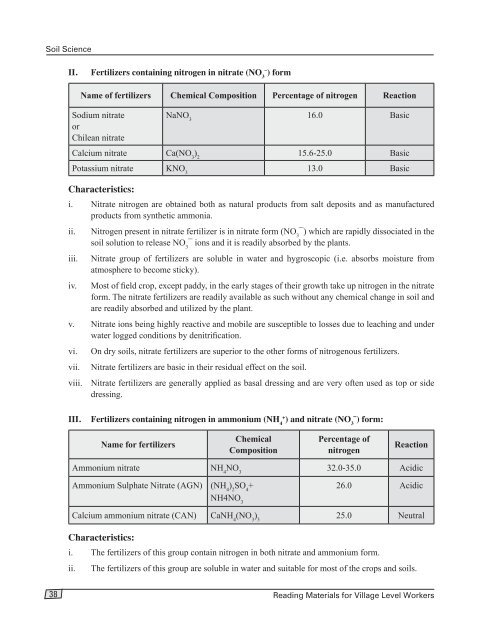
Fertilizers containing nitrogen in nitrate (NO 3 – ) form<br />
Name of fertilizers Chemical Composition Percentage of nitrogen Reaction<br />
Sodium nitrate<br />
or<br />
Chilean nitrate<br />
NaNO 3<br />
16.0 Basic<br />
Calcium nitrate Ca(NO 3<br />
) 2<br />
15.6-25.0 Basic<br />
Potassium nitrate KNO 3<br />
13.0 Basic<br />
Characteristics:<br />
i. Nitrate nitrogen are obtained both as natural products from salt deposits and as manufactured<br />
products from synthetic ammonia.<br />
ii.<br />
iii.<br />
iv.<br />
Nitrogen present in nitrate fertilizer is in nitrate form (NO 3¯) which are rapidly dissociated in the<br />
soil solution to release NO 3¯ ions and it is readily absorbed by the plants.<br />
Nitrate group of fertilizers are soluble in water and hygroscopic (i.e. absorbs moisture from<br />
atmosphere to become sticky).<br />
Most of field crop, except paddy, in the early stages of their growth take up nitrogen in the nitrate<br />
form. The nitrate fertilizers are readily available as such without any chemical change in soil and<br />
are readily absorbed and utilized by the plant.<br />
v. Nitrate ions being highly reactive and mobile are susceptible to losses due to leaching and under<br />
water logged conditions by denitrification.<br />
vi.<br />
vii.<br />
On dry soils, nitrate fertilizers are superior to the other forms of nitrogenous fertilizers.<br />
Nitrate fertilizers are basic in their residual effect on the soil.<br />
viii. Nitrate fertilizers are generally applied as basal dressing and are very often used as top or side<br />
dressing.<br />
III.<br />
Fertilizers containing nitrogen in ammonium (NH 4+<br />
) and nitrate (NO 3¯) form:<br />
Name for fertilizers<br />
Chemical<br />
Composition<br />
Percentage of<br />
nitrogen<br />
Reaction<br />
Ammonium nitrate NH 4<br />
NO 3<br />
32.0-35.0 Acidic<br />
Ammonium Sulphate Nitrate (AGN) (NH 4<br />
) 2<br />
SO 4<br />
+<br />
NH4NO 3<br />
26.0 Acidic<br />
Calcium ammonium nitrate (CAN) CaNH 4<br />
(NO 3<br />
) 3<br />
25.0 Neutral<br />
Characteristics:<br />
i. The fertilizers of this group contain nitrogen in both nitrate and ammonium form.<br />
ii.<br />
The fertilizers of this group are soluble in water and suitable for most of the crops and soils.<br />
38 Reading Materials for Village Level Workers