Health Canada Module 1 Updates and Progess Towards ... - TOPRA
Health Canada Module 1 Updates and Progess Towards ... - TOPRA
Health Canada Module 1 Updates and Progess Towards ... - TOPRA
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
much further along in the implementation electronic submission process <strong>and</strong> has a wealth of experience to<br />
share. In a time of budget cuts that have affected both countries regulatory bodies, modernizing the<br />
regulatory <strong>and</strong> policy process in a way that fosters collaboration both now <strong>and</strong> in the future has the<br />
potential to not only reduce costs but to increase quality <strong>and</strong> safety in the process. Careful attention must<br />
be paid to ensure the st<strong>and</strong>ards adopted through harmonization do not result in a lowering of the current<br />
guidelines.<br />
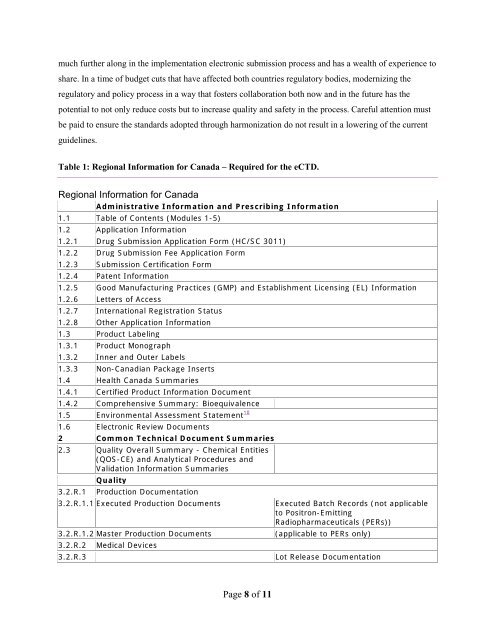
Table 1: Regional Information for <strong>Canada</strong> – Required for the eCTD.<br />
Regional Information for <strong>Canada</strong><br />
Administrative Information <strong>and</strong> Prescribing Information<br />
1.1 Table of Contents (<strong>Module</strong>s 1-5)<br />
1.2 Application Information<br />
1.2.1 Drug Submission Application Form (HC/SC 3011)<br />
1.2.2 Drug Submission Fee Application Form<br />
1.2.3 Submission Certification Form<br />
1.2.4 Patent Information<br />
1.2.5 Good Manufacturing Practices (GMP) <strong>and</strong> Establishment Licensing (EL) Information<br />
1.2.6 Letters of Access<br />
1.2.7 International Registration Status<br />
1.2.8 Other Application Information<br />
1.3 Product Labeling<br />
1.3.1 Product Monograph<br />
1.3.2 Inner <strong>and</strong> Outer Labels<br />
1.3.3 Non-Canadian Package Inserts<br />
1.4 <strong>Health</strong> <strong>Canada</strong> Summaries<br />
1.4.1 Certified Product Information Document<br />
1.4.2 Comprehensive Summary: Bioequivalence<br />
1.5 Environmental Assessment Statement 10<br />
1.6 Electronic Review Documents<br />
2 Common Technical Document Summaries<br />
2.3 Quality Overall Summary - Chemical Entities<br />
(QOS-CE) <strong>and</strong> Analytical Procedures <strong>and</strong><br />
Validation Information Summaries<br />
3.2.R.1<br />
Quality<br />
Production Documentation<br />
3.2.R.1.1 Executed Production Documents<br />
3.2.R.1.2 Master Production Documents<br />
3.2.R.2<br />
3.2.R.3<br />
Medical Devices<br />
Executed Batch Records (not applicable<br />
to Positron-Emitting<br />
Radiopharmaceuticals (PERs))<br />
(applicable to PERs only)<br />
Lot Release Documentation<br />
Page 8 of 11