#10: The Discovery of Hydrogen Isotopes
#10: The Discovery of Hydrogen Isotopes
#10: The Discovery of Hydrogen Isotopes
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
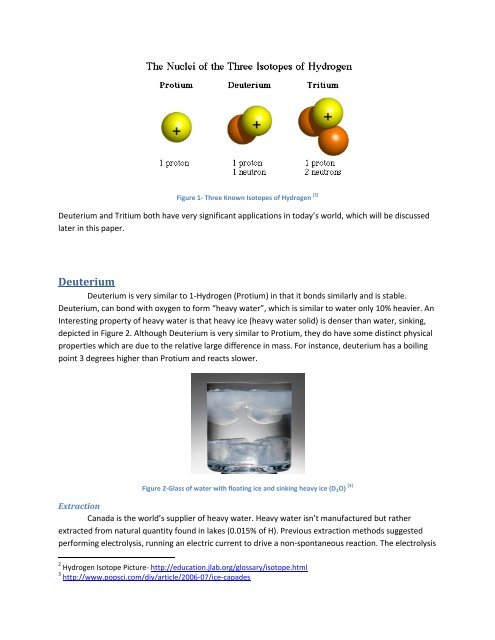
Figure 1- Three Known <strong>Isotopes</strong> <strong>of</strong> <strong>Hydrogen</strong> [2]<br />
Deuterium and Tritium both have very significant applications in today’s world, which will be discussed<br />
later in this paper.<br />
Deuterium<br />
Deuterium is very similar to 1-<strong>Hydrogen</strong> (Protium) in that it bonds similarly and is stable.<br />
Deuterium, can bond with oxygen to form “heavy water”, which is similar to water only 10% heavier. An<br />
Interesting property <strong>of</strong> heavy water is that heavy ice (heavy water solid) is denser than water, sinking,<br />
depicted in Figure 2. Although Deuterium is very similar to Protium, they do have some distinct physical<br />
properties which are due to the relative large difference in mass. For instance, deuterium has a boiling<br />
point 3 degrees higher than Protium and reacts slower.<br />
Figure 2-Glass <strong>of</strong> water with floating ice and sinking heavy ice (D 2 O) [3]<br />
Extraction<br />
Canada is the world’s supplier <strong>of</strong> heavy water. Heavy water isn’t manufactured but rather<br />
extracted from natural quantity found in lakes (0.015% <strong>of</strong> H). Previous extraction methods suggested<br />
performing electrolysis, running an electric current to drive a non-spontaneous reaction. <strong>The</strong> electrolysis<br />
2 <strong>Hydrogen</strong> Isotope Picture- http://education.jlab.org/glossary/isotope.html<br />
3 http://www.popsci.com/diy/article/2006-07/ice-capades