2PHA206 Chemistry for Pharmacy (II) - James Smith
2PHA206 Chemistry for Pharmacy (II) - James Smith
2PHA206 Chemistry for Pharmacy (II) - James Smith
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
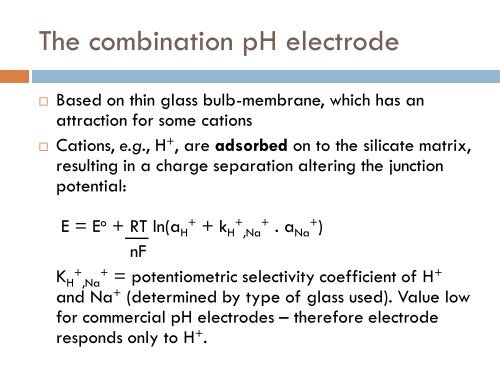
The combination pH electrode<br />
Based on thin glass bulb-membrane, which has an<br />
attraction <strong>for</strong> some cations<br />
Cations, e.g., H + , are adsorbed on to the silicate matrix,<br />
resulting in a charge separation altering the junction<br />
potential:<br />
E = E o + RT ln(a H<br />
+<br />
+ k H<br />
+<br />
,Na<br />
+<br />
. a Na+ )<br />
nF<br />
K H<br />
+<br />
,Na<br />
+<br />
= potentiometric selectivity coefficient of H +<br />
and Na + (determined by type of glass used). Value low<br />
<strong>for</strong> commercial pH electrodes – there<strong>for</strong>e electrode<br />
responds only to H + .