Structural Diversity of Ethinyl Estradiol Solvates
Structural Diversity of Ethinyl Estradiol Solvates
Structural Diversity of Ethinyl Estradiol Solvates
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Structural</strong> <strong>Diversity</strong> <strong>of</strong> <strong>Ethinyl</strong> <strong>Estradiol</strong> <strong>Solvates</strong> Crystal Growth & Design, Vol. 8, No. 3, 2008 829<br />
Table 4. <strong>Structural</strong> Data for All Pseudo-Polymorphic Forms <strong>of</strong> <strong>Ethinyl</strong> <strong>Estradiol</strong><br />
pseudo-polymorphs type <strong>of</strong> H-bond packing patterns <strong>of</strong> EE molecules H-bond network EE/solvent ratio<br />
donor/acceptor (D/A)<br />
propensity <strong>of</strong> the<br />
solvent molecules<br />
hemihydrate C(3)-OH ···X a head-to-tail 2D 1/2 D/A<br />
C(17)-OH ···X a<br />
C(3) ···OH ···C(17)<br />
methanolate C(3)-OH ···X a head-to-tail-to-methanol 2D 1 D/A<br />
C(17)-OH ···X a<br />
C(3) ···OH ···C(17)<br />
ACN C(3)-OH ···X a head-to-head-to-tail 2D 2 D/A<br />
C(17)-OH ···X a<br />
C(3) ···OH ···C(17)<br />
C(3) ···OH ···C(33)<br />
dioxane C(17)-OH ···X a head-to-tail 1D 1 A<br />
C(3) ···OH ···C(17)<br />
C(3) ···OH ···C(33)<br />
ethinyl ···X a<br />
nitromethane C(17)-OH ···X a head-to-tail 2D 2 A<br />
C(3) ···OH ···C(17)<br />
C(3) ···OH ···C(33)<br />
ethanolate C(3)-OH ···X a head-to-tail-to-ethanol-to-tail 3D 3/2 D/A<br />
C(17)-OH ···a<br />
C(3) ···OH ···C(17)<br />
ethinyl ···O-C(33)<br />
DMF C(3)-OH ···X a EE-to-DMF 0D 1/2 A<br />
C(17)-OH ···X a<br />
C(18)-H (18) ···O(17)<br />
X a ···X a<br />
a From the solvent molecules.<br />
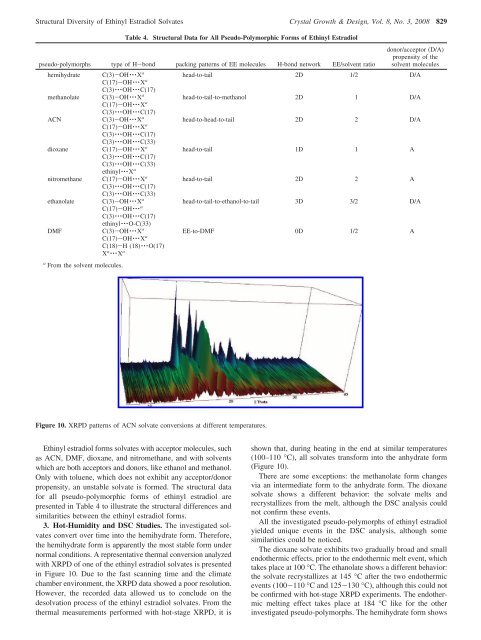
Figure 10. XRPD patterns <strong>of</strong> ACN solvate conversions at different temperatures.<br />
<strong>Ethinyl</strong> estradiol forms solvates with acceptor molecules, such<br />
as ACN, DMF, dioxane, and nitromethane, and with solvents<br />
which are both acceptors and donors, like ethanol and methanol.<br />
Only with toluene, which does not exhibit any acceptor/donor<br />
propensity, an unstable solvate is formed. The structural data<br />
for all pseudo-polymorphic forms <strong>of</strong> ethinyl estradiol are<br />
presented in Table 4 to illustrate the structural differences and<br />
similarities between the ethinyl estradiol forms.<br />
3. Hot-Humidity and DSC Studies. The investigated solvates<br />
convert over time into the hemihydrate form. Therefore,<br />
the hemihydrate form is apparently the most stable form under<br />
normal conditions. A representative thermal conversion analyzed<br />
with XRPD <strong>of</strong> one <strong>of</strong> the ethinyl estradiol solvates is presented<br />
in Figure 10. Due to the fast scanning time and the climate<br />
chamber environment, the XRPD data showed a poor resolution.<br />
However, the recorded data allowed us to conclude on the<br />
desolvation process <strong>of</strong> the ethinyl estradiol solvates. From the<br />
thermal measurements performed with hot-stage XRPD, it is<br />
shown that, during heating in the end at similar temperatures<br />
(100–110 °C), all solvates transform into the anhydrate form<br />
(Figure 10).<br />
There are some exceptions: the methanolate form changes<br />
via an intermediate form to the anhydrate form. The dioxane<br />
solvate shows a different behavior: the solvate melts and<br />
recrystallizes from the melt, although the DSC analysis could<br />
not confirm these events.<br />
All the investigated pseudo-polymorphs <strong>of</strong> ethinyl estradiol<br />
yielded unique events in the DSC analysis, although some<br />
similarities could be noticed.<br />
The dioxane solvate exhibits two gradually broad and small<br />
endothermic effects, prior to the endothermic melt event, which<br />
takes place at 100 °C. The ethanolate shows a different behavior:<br />
the solvate recrystallizes at 145 °C after the two endothermic<br />
events (100-110 °C and 125-130 °C), although this could not<br />
be confirmed with hot-stage XRPD experiments. The endothermic<br />
melting effect takes place at 184 °C like for the other<br />
investigated pseudo-polymorphs. The hemihydrate form shows