Division of Medicinal Chemistry Abstracts-235th ACS National ...
Division of Medicinal Chemistry Abstracts-235th ACS National ...
Division of Medicinal Chemistry Abstracts-235th ACS National ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
MEDI 72<br />
Synthesis and SAR studies <strong>of</strong> a novel series <strong>of</strong> T-type calcium channel blockers<br />
Yun Jeong Choe 1 , Han Na Seo 1 , Ja Youn Choi 1 , Jungahn Kim 2 , Dong Joon Choo 2 , and Jae<br />
Yeol Lee 2 . (1) Department <strong>of</strong> <strong>Chemistry</strong>, Kyung Hee University, 1 Hoegi-dong, Dongdaemoongu,<br />
Seoul 130-701, South Korea, Fax: 82-2-966-3701, whitebear48@naver.com, (2)<br />
Department <strong>of</strong> <strong>Chemistry</strong>, Kyunghee University, Seoul 130-701, South Korea<br />
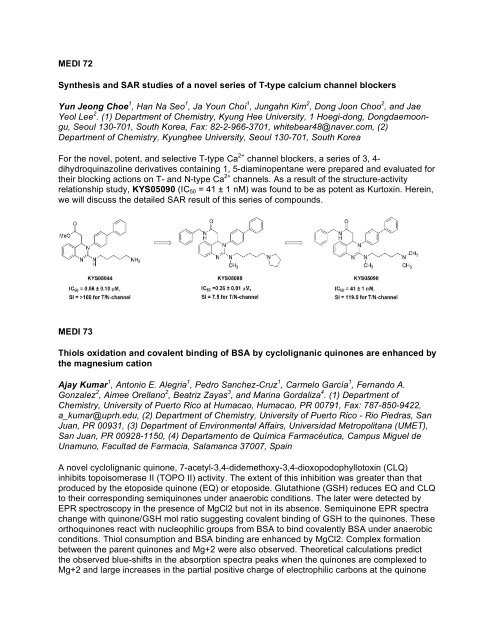
For the novel, potent, and selective T-type Ca 2+ channel blockers, a series <strong>of</strong> 3, 4-<br />
dihydroquinazoline derivatives containing 1, 5-diaminopentane were prepared and evaluated for<br />
their blocking actions on T- and N-type Ca 2+ channels. As a result <strong>of</strong> the structure-activity<br />
relationship study, KYS05090 (IC 50 = 41 ± 1 nM) was found to be as potent as Kurtoxin. Herein,<br />
we will discuss the detailed SAR result <strong>of</strong> this series <strong>of</strong> compounds.<br />
MEDI 73<br />
Thiols oxidation and covalent binding <strong>of</strong> BSA by cyclolignanic quinones are enhanced by<br />
the magnesium cation<br />
Ajay Kumar 1 , Antonio E. Alegria 1 , Pedro Sanchez-Cruz 1 , Carmelo García 1 , Fernando A.<br />
Gonzalez 2 , Aimee Orellano 2 , Beatriz Zayas 3 , and Marina Gordaliza 4 . (1) Department <strong>of</strong><br />
<strong>Chemistry</strong>, University <strong>of</strong> Puerto Rico at Humacao, Humacao, PR 00791, Fax: 787-850-9422,<br />
a_kumar@uprh.edu, (2) Department <strong>of</strong> <strong>Chemistry</strong>, University <strong>of</strong> Puerto Rico - Rio Piedras, San<br />
Juan, PR 00931, (3) Department <strong>of</strong> Environmental Affairs, Universidad Metropolitana (UMET),<br />
San Juan, PR 00928-1150, (4) Departamento de Química Farmacéutica, Campus Miguel de<br />
Unamuno, Facultad de Farmacia, Salamanca 37007, Spain<br />
A novel cyclolignanic quinone, 7-acetyl-3,4-didemethoxy-3,4-dioxopodophyllotoxin (CLQ)<br />
inhibits topoisomerase II (TOPO II) activity. The extent <strong>of</strong> this inhibition was greater than that<br />
produced by the etoposide quinone (EQ) or etoposide. Glutathione (GSH) reduces EQ and CLQ<br />
to their corresponding semiquinones under anaerobic conditions. The later were detected by<br />
EPR spectroscopy in the presence <strong>of</strong> MgCl2 but not in its absence. Semiquinone EPR spectra<br />
change with quinone/GSH mol ratio suggesting covalent binding <strong>of</strong> GSH to the quinones. These<br />
orthoquinones react with nucleophilic groups from BSA to bind covalently BSA under anaerobic<br />
conditions. Thiol consumption and BSA binding are enhanced by MgCl2. Complex formation<br />
between the parent quinones and Mg+2 were also observed. Theoretical calculations predict<br />
the observed blue-shifts in the absorption spectra peaks when the quinones are complexed to<br />
Mg+2 and large increases in the partial positive charge <strong>of</strong> electrophilic carbons at the quinone