Osmotic Swelling of Polyacrylate Hydrogels in Physiological Salt ...
Osmotic Swelling of Polyacrylate Hydrogels in Physiological Salt ...
Osmotic Swelling of Polyacrylate Hydrogels in Physiological Salt ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Gel <strong>Swell<strong>in</strong>g</strong> <strong>in</strong> <strong>Physiological</strong> <strong>Salt</strong> Solutions Biomacromolecules, Vol. 1, No. 1, 2000 89<br />
The molecular mechanism that causes the observed<br />
modification <strong>of</strong> the χ parameter is not entirely clear.<br />
Experimental results suggest that there are specific <strong>in</strong>teractions<br />
between the network and the Ca 2+ ions. The Ca 2+ may<br />
also <strong>in</strong>fluence the flexibility <strong>of</strong> the polymer cha<strong>in</strong>s. In<br />
general, divalent cations promote the formation <strong>of</strong> aggregates<br />
because <strong>of</strong> their high ion valence and small hydrodynamic<br />
radius. In highly swollen gels the attraction between charged<br />
cha<strong>in</strong>s may lead to formation <strong>of</strong> bundles. S<strong>in</strong>ce the elastic<br />
properties <strong>of</strong> these gels are not <strong>in</strong>fluenced considerably by<br />
the Ca 2+ ions, the aggregates should be loose. This picture<br />
is qualitatively consistent with the <strong>in</strong>dependence <strong>of</strong> the elastic<br />
modulus <strong>of</strong> the salt concentration and also with the observed<br />
<strong>in</strong>crease <strong>of</strong> the χ parameter with <strong>in</strong>creas<strong>in</strong>g Ca 2+ concentration.<br />
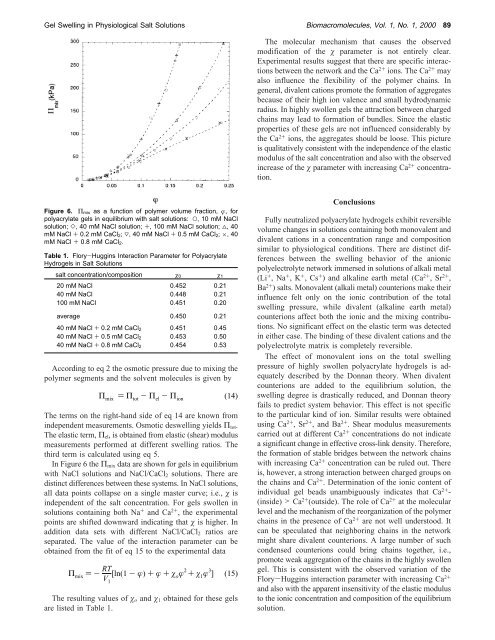
Figure 6. Π mix as a function <strong>of</strong> polymer volume fraction, φ, for<br />
polyacrylate gels <strong>in</strong> equilibrium with salt solutions: O, 10 mM NaCl<br />
solution; ], 40 mM NaCl solution; +, 100 mM NaCl solution; 4, 40<br />
mM NaCl + 0.2 mM CaCl 2; 3, 40 mM NaCl + 0.5 mM CaCl 2; ×, 40<br />
mM NaCl + 0.8 mM CaCl 2.<br />
Table 1. Flory-Hugg<strong>in</strong>s Interaction Parameter for <strong>Polyacrylate</strong><br />
<strong>Hydrogels</strong> <strong>in</strong> <strong>Salt</strong> Solutions<br />
salt concentration/composition χ 0 χ 1<br />
20 mM NaCl 0.452 0.21<br />
40 mM NaCl 0.448 0.21<br />
100 mM NaCl 0.451 0.20<br />
average 0.450 0.21<br />
40 mM NaCl + 0.2 mM CaCl 2 0.451 0.45<br />
40 mM NaCl + 0.5 mM CaCl 2 0.453 0.50<br />
40 mM NaCl + 0.8 mM CaCl 2 0.454 0.53<br />
Accord<strong>in</strong>g to eq 2 the osmotic pressure due to mix<strong>in</strong>g the<br />
polymer segments and the solvent molecules is given by<br />
Π mix ) Π tot - Π el - Π ion (14)<br />
The terms on the right-hand side <strong>of</strong> eq 14 are known from<br />
<strong>in</strong>dependent measurements. <strong>Osmotic</strong> deswell<strong>in</strong>g yields Π tot .<br />
The elastic term, Π el , is obta<strong>in</strong>ed from elastic (shear) modulus<br />
measurements performed at different swell<strong>in</strong>g ratios. The<br />
third term is calculated us<strong>in</strong>g eq 5.<br />
In Figure 6 the Π mix data are shown for gels <strong>in</strong> equilibrium<br />
with NaCl solutions and NaCl/CaCl 2 solutions. There are<br />
dist<strong>in</strong>ct differences between these systems. In NaCl solutions,<br />
all data po<strong>in</strong>ts collapse on a s<strong>in</strong>gle master curve; i.e., χ is<br />
<strong>in</strong>dependent <strong>of</strong> the salt concentration. For gels swollen <strong>in</strong><br />
solutions conta<strong>in</strong><strong>in</strong>g both Na + and Ca 2+ , the experimental<br />
po<strong>in</strong>ts are shifted downward <strong>in</strong>dicat<strong>in</strong>g that χ is higher. In<br />
addition data sets with different NaCl/CaCl 2 ratios are<br />
separated. The value <strong>of</strong> the <strong>in</strong>teraction parameter can be<br />
obta<strong>in</strong>ed from the fit <strong>of</strong> eq 15 to the experimental data<br />
Π mix )- RT<br />
V 1<br />
[ln(1 - φ) + φ + χ ο φ 2 + χ 1 φ 3 ] (15)<br />
The result<strong>in</strong>g values <strong>of</strong> χ ο and χ 1 obta<strong>in</strong>ed for these gels<br />
are listed <strong>in</strong> Table 1.<br />
Conclusions<br />
Fully neutralized polyacrylate hydrogels exhibit reversible<br />
volume changes <strong>in</strong> solutions conta<strong>in</strong><strong>in</strong>g both monovalent and<br />
divalent cations <strong>in</strong> a concentration range and composition<br />
similar to physiological conditions. There are dist<strong>in</strong>ct differences<br />
between the swell<strong>in</strong>g behavior <strong>of</strong> the anionic<br />
polyelectrolyte network immersed <strong>in</strong> solutions <strong>of</strong> alkali metal<br />
(Li + ,Na + ,K + ,Cs + ) and alkal<strong>in</strong>e earth metal (Ca 2+ ,Sr 2+ ,<br />
Ba 2+ ) salts. Monovalent (alkali metal) counterions make their<br />
<strong>in</strong>fluence felt only on the ionic contribution <strong>of</strong> the total<br />
swell<strong>in</strong>g pressure, while divalent (alkal<strong>in</strong>e earth metal)<br />
counterions affect both the ionic and the mix<strong>in</strong>g contributions.<br />
No significant effect on the elastic term was detected<br />
<strong>in</strong> either case. The b<strong>in</strong>d<strong>in</strong>g <strong>of</strong> these divalent cations and the<br />
polyelectrolyte matrix is completely reversible.<br />
The effect <strong>of</strong> monovalent ions on the total swell<strong>in</strong>g<br />
pressure <strong>of</strong> highly swollen polyacrylate hydrogels is adequately<br />
described by the Donnan theory. When divalent<br />
counterions are added to the equilibrium solution, the<br />
swell<strong>in</strong>g degree is drastically reduced, and Donnan theory<br />
fails to predict system behavior. This effect is not specific<br />
to the particular k<strong>in</strong>d <strong>of</strong> ion. Similar results were obta<strong>in</strong>ed<br />
us<strong>in</strong>g Ca 2+ ,Sr 2+ , and Ba 2+ . Shear modulus measurements<br />
carried out at different Ca 2+ concentrations do not <strong>in</strong>dicate<br />
a significant change <strong>in</strong> effective cross-l<strong>in</strong>k density. Therefore,<br />
the formation <strong>of</strong> stable bridges between the network cha<strong>in</strong>s<br />
with <strong>in</strong>creas<strong>in</strong>g Ca 2+ concentration can be ruled out. There<br />
is, however, a strong <strong>in</strong>teraction between charged groups on<br />
the cha<strong>in</strong>s and Ca 2+ . Determ<strong>in</strong>ation <strong>of</strong> the ionic content <strong>of</strong><br />
<strong>in</strong>dividual gel beads unambiguously <strong>in</strong>dicates that Ca 2+ -<br />
(<strong>in</strong>side) > Ca 2+ (outside). The role <strong>of</strong> Ca 2+ at the molecular<br />
level and the mechanism <strong>of</strong> the reorganization <strong>of</strong> the polymer<br />
cha<strong>in</strong>s <strong>in</strong> the presence <strong>of</strong> Ca 2+ are not well understood. It<br />
can be speculated that neighbor<strong>in</strong>g cha<strong>in</strong>s <strong>in</strong> the network<br />
might share divalent counterions. A large number <strong>of</strong> such<br />
condensed counterions could br<strong>in</strong>g cha<strong>in</strong>s together, i.e.,<br />
promote weak aggregation <strong>of</strong> the cha<strong>in</strong>s <strong>in</strong> the highly swollen<br />
gel. This is consistent with the observed variation <strong>of</strong> the<br />
Flory-Hugg<strong>in</strong>s <strong>in</strong>teraction parameter with <strong>in</strong>creas<strong>in</strong>g Ca 2+<br />
and also with the apparent <strong>in</strong>sensitivity <strong>of</strong> the elastic modulus<br />
to the ionic concentration and composition <strong>of</strong> the equilibrium<br />
solution.