Medical Device Testing Guide - Toxikon Corporation
Medical Device Testing Guide - Toxikon Corporation
Medical Device Testing Guide - Toxikon Corporation
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
SUBCHRONIC SYSTEMIC TOXICITY TESTING<br />
Rev. May 2011<br />
AF03-1<br />
28-Day IV Injection Sub-Chronic Toxicity Study- Sample needed: To be determined, if < 0.5 mm thick 20,160 cm 2 or 672g<br />
Approximate turnaround time is twelve to fourteen weeks.<br />
AF03-2<br />
Abridged-28 Day IV Dose Toxicity Study- Sample needed: To be determined, if > 0.5 mm thick 10,080 cm 2 or 336g<br />
Approximate turnaround time is eleven weeks.<br />
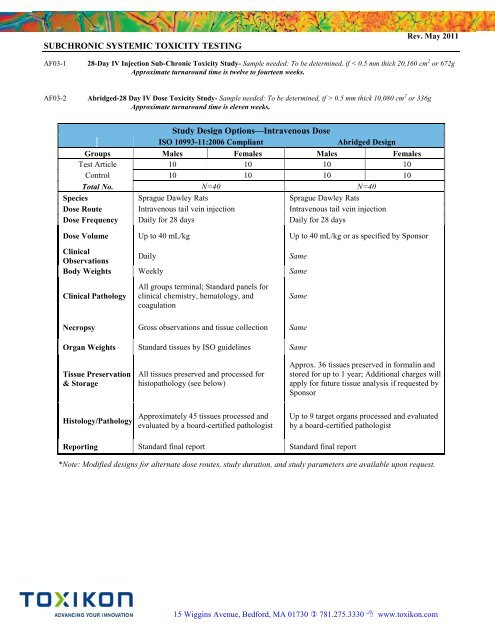
Study Design Options—Intravenous Dose<br />
ISO 10993-11:2006 Compliant<br />
Abridged Design<br />
Groups Males Females Males Females<br />
Test Article 10 10 10 10<br />
Control 10 10 10 10<br />
Total No. N=40 N=40<br />
Species Sprague Dawley Rats Sprague Dawley Rats<br />
Dose Route Intravenous tail vein injection Intravenous tail vein injection<br />
Dose Frequency Daily for 28 days Daily for 28 days<br />
Dose Volume Up to 40 mL/kg Up to 40 mL/kg or as specified by Sponsor<br />
Clinical<br />
Observations<br />
Daily<br />
Same<br />
Body Weights Weekly Same<br />
Clinical Pathology<br />
All groups terminal; Standard panels for<br />
clinical chemistry, hematology, and<br />
coagulation<br />
Same<br />
Necropsy Gross observations and tissue collection Same<br />
Organ Weights Standard tissues by ISO guidelines Same<br />
Tissue Preservation<br />
& Storage<br />
All tissues preserved and processed for<br />
histopathology (see below)<br />
Approx. 36 tissues preserved in formalin and<br />
stored for up to 1 year; Additional charges will<br />
apply for future tissue analysis if requested by<br />
Sponsor<br />
Histology/Pathology<br />
Approximately 45 tissues processed and<br />
evaluated by a board-certified pathologist<br />
Up to 9 target organs processed and evaluated<br />
by a board-certified pathologist<br />
Reporting Standard final report Standard final report<br />
*Note: Modified designs for alternate dose routes, study duration, and study parameters are available upon request.<br />
15 Wiggins Avenue, Bedford, MA 01730 781.275.3330 www.toxikon.com