Excel
Excel
Excel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
C18<br />
Mechanisms of<br />
Separation<br />
Hydrophobic binding<br />
interactions<br />
Shape selectivity<br />
Target Analytes<br />
Analytes differing in hydrophobicity<br />
Polar, moderately polar and nonpolar analytes<br />
Uncharged acids and bases<br />
Ionized acids or bases using ion-pairing<br />
Recommended Applications<br />
Analytes differing in hydrophobicity<br />
Strength of<br />
Interaction<br />
Strong<br />
Weak<br />
Homologous compounds differing by –CH2<br />
Ideal starting point for method development<br />
Further Information;<br />
An HPLC product brochure discussing ACE C18 and all<br />
ACE phases is available.<br />
Contact your distributor to request your copy or visit<br />
www.ace-hplc.com for further details.<br />
6<br />
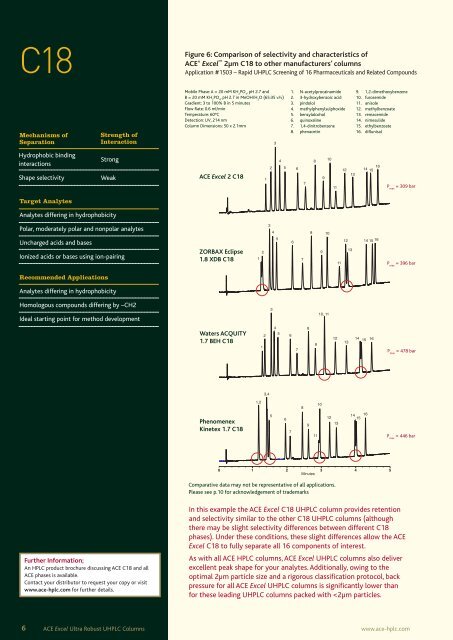
Figure 6: Comparison of selectivity and characteristics of<br />
ACE ® <strong>Excel</strong> TM<br />
2µm C18 to other manufacturers’ columns<br />
Application #1503 – Rapid UHPLC Screening of 16 Pharmaceuticals and Related Compounds<br />
Mobile Phase: A = 20 mM KH 2 PO 4 , pH 2.7 and<br />
B = 20 mM KH 2 PO 4 , pH 2.7 in MeOH/H 2 O (65:35 v/v)<br />
Gradient: 3 to 100% B in 5 minutes<br />
Flow Rate: 0.6 ml/min<br />
Temperature: 60°C<br />
Detection: UV, 214 nm<br />
Column Dimensions: 50 x 2.1mm<br />
ACE <strong>Excel</strong> 2 C18<br />
ZORBAX Eclipse<br />
1.8 XDB C18<br />
Waters ACQUITY<br />
1.7 BEH C18<br />
Phenomenex<br />
Kinetex 1.7 C18<br />
0<br />
1<br />
2<br />
1<br />
1,2<br />
1<br />
1. N-acetylprocainamide<br />
2. 3-hydroxybenzoic acid<br />
3. pindolol<br />
4. methylphenylsulphoxide<br />
5. benzylalcohol<br />
6. quinoxaline<br />
7. 1,4-dinitrobenzene<br />
8. phenacetin<br />
Comparative data may not be representative of all applications.<br />
Please see p.10 for acknowledgement of trademarks<br />
2<br />
1<br />
3,4<br />
2<br />
3<br />
4<br />
5<br />
3<br />
5<br />
3<br />
4<br />
5<br />
4<br />
5<br />
6<br />
2<br />
6<br />
7<br />
6<br />
6<br />
7<br />
Minutes<br />
9. 1,2-dimethoxybenzene<br />
10. furosemide<br />
11. anisole<br />
12. methylbenzoate<br />
13. remacemide<br />
14. nimesulide<br />
15. ethylbenzoate<br />
16. diflunisal<br />
In this example the ACE <strong>Excel</strong> C18 UHPLC column provides retention<br />
and selectivity similar to the other C18 UHPLC columns (although<br />
there may be slight selectivity differences between different C18<br />
phases). Under these conditions, these slight differences allow the ACE<br />
<strong>Excel</strong> C18 to fully separate all 16 components of interest.<br />
As with all ACE HPLC columns, ACE <strong>Excel</strong> UHPLC columns also deliver<br />
excellent peak shape for your analytes. Additionally, owing to the<br />
optimal 2µm particle size and a rigorous classification protocol, back<br />
pressure for all ACE <strong>Excel</strong> UHPLC columns is significantly lower than<br />
for these leading UHPLC columns packed with