ACID BASE BALANCE
ACID BASE BALANCE
ACID BASE BALANCE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
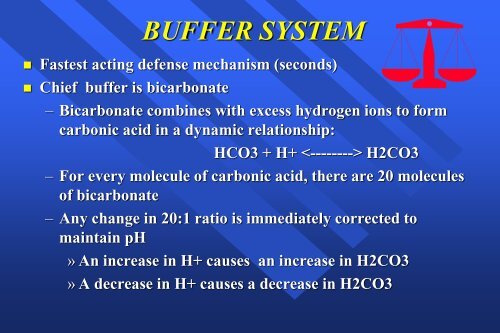
BUFFER SYSTEM<br />
• Fastest acting defense mechanism (seconds)<br />
• Chief buffer is bicarbonate<br />
– Bicarbonate combines with excess hydrogen ions to form<br />
carbonic acid in a dynamic relationship:<br />
HCO3 + H+ H2CO3<br />
– For every molecule of carbonic acid, there are 20 molecules<br />
of bicarbonate<br />
– Any change in 20:1 ratio is immediately corrected to<br />
maintain pH<br />
» An increase in H+ causes an increase in H2CO3<br />
» A decrease in H+ causes a decrease in H2CO3