Structure and Properties of Neat Liquids Using Nonadditive ...
Structure and Properties of Neat Liquids Using Nonadditive ...
Structure and Properties of Neat Liquids Using Nonadditive ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Structure</strong> <strong>and</strong> <strong>Properties</strong> <strong>of</strong> <strong>Neat</strong> <strong>Liquids</strong> J. Phys. Chem., Vol. 99, No. 16, 1995 6211<br />
Water Neutron Diffraction<br />
C<br />
.- 0<br />
c<br />
0<br />
C<br />
3<br />
U<br />
c<br />
3.0 -<br />
.- 0<br />
c<br />
2 2.0<br />
.-<br />
L c<br />
-<br />
n<br />
II<br />
El<br />
...........<br />
I HHJ<br />
I<br />
0.0<br />
distance (Angstroms)<br />
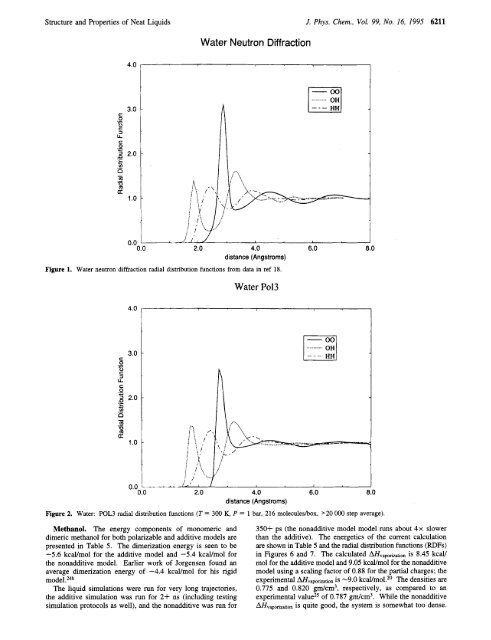
Figure 1. Water neutron diffraction radial distribution functions from data in ref 18.<br />
Water Po13<br />
4.0<br />
c<br />
.- 0<br />
c<br />
0<br />
t 3<br />
U<br />
c<br />
.- 0<br />
3.0<br />
I<br />
2 2.0<br />
1-<br />
c L<br />
v)<br />
6 -<br />
.- m<br />
'0<br />
2<br />
1 .o<br />
n<br />
r;3 ............<br />
...........<br />
0.0<br />
distance (Angstroms)<br />
Figure 2. Water: POL3 radial distribution functions (T = 300 K, P = 1 bar, 216 moleculesbox, '20 000 step average).<br />
Methanol. The energy components <strong>of</strong> monomeric <strong>and</strong><br />
dimeric methanol for both polarizable <strong>and</strong> additive models are<br />
presented in Table 5. The dimerization energy is seen to be<br />
-5.6 kcal/mol for the additive model <strong>and</strong> -5.4 kcaVmol for<br />
the nonadditive model. Earlier work <strong>of</strong> Jorgensen found an<br />
average dimerization energy <strong>of</strong> -4.4 kcal/mol for his rigid<br />
The liquid simulations were run for very long trajectories,<br />
the additive simulation was run for 2+ ns (including testing<br />
simulation protocols as well), <strong>and</strong> the nonadditive was run for<br />
350f ps (the nonadditive model model runs about 4x slower<br />
than the additive). The energetics <strong>of</strong> the current calculation<br />
are shown in Table 5 <strong>and</strong> the radial distribution functions (RDFs)<br />
in Figures 6 <strong>and</strong> 7. The calculated AHvapoization is 8.45 kcaV<br />
mol for the additive model <strong>and</strong> 9.05 kcal/mol for the nonadditive<br />
model using a scaling factor <strong>of</strong> 0.88 for the partial charges; the<br />
experimental AHvap<strong>of</strong>ization is -9.0 kcal/mol.20 The densities are<br />
0.775 <strong>and</strong> 0.820 gm/cm3, respectively, as compared to an<br />
experimental value25 <strong>of</strong> 0.787 gm/cm3. While the nonadditive<br />
AHvapo"zation is quite good, the system is somewhat too dense.