Paper Chromatography Separation of Inorganic Cations
Paper Chromatography Separation of Inorganic Cations
Paper Chromatography Separation of Inorganic Cations
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
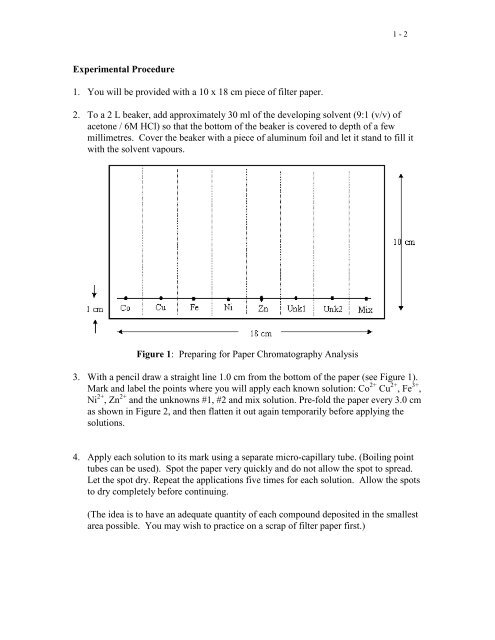
1 - 2Experimental Procedure1. You will be provided with a 10 x 18 cm piece <strong>of</strong> filter paper.2. To a 2 L beaker, add approximately 30 ml <strong>of</strong> the developing solvent (9:1 (v/v) <strong>of</strong>acetone / 6M HCl) so that the bottom <strong>of</strong> the beaker is covered to depth <strong>of</strong> a fewmillimetres. Cover the beaker with a piece <strong>of</strong> aluminum foil and let it stand to fill itwith the solvent vapours.Figure 1: Preparing for <strong>Paper</strong> <strong>Chromatography</strong> Analysis3. With a pencil draw a straight line 1.0 cm from the bottom <strong>of</strong> the paper (see Figure 1).Mark and label the points where you will apply each known solution: Co 2+ Cu 2+ , Fe 3+ ,Ni 2+ , Zn 2+ and the unknowns #1, #2 and mix solution. Pre-fold the paper every 3.0 cmas shown in Figure 2, and then flatten it out again temporarily before applying thesolutions.4. Apply each solution to its mark using a separate micro-capillary tube. (Boiling pointtubes can be used). Spot the paper very quickly and do not allow the spot to spread.Let the spot dry. Repeat the applications five times for each solution. Allow the spotsto dry completely before continuing.(The idea is to have an adequate quantity <strong>of</strong> each compound deposited in the smallestarea possible. You may wish to practice on a scrap <strong>of</strong> filter paper first.)