Carbonyl Reactions - Moravian College Chemistry Department
Carbonyl Reactions - Moravian College Chemistry Department
Carbonyl Reactions - Moravian College Chemistry Department
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
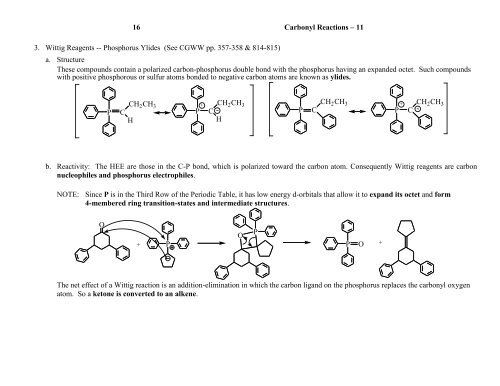
16 <strong>Carbonyl</strong> <strong>Reactions</strong> – 113. Wittig Reagents -- Phosphorus Ylides (See CGWW pp. 357-358 & 814-815)a. StructureThese compounds contain a polarized carbon-phosphorus double bond with the phosphorus having an expanded octet. Such compoundswith positive phosphorous or sulfur atoms bonded to negative carbon atoms are known as ylides.PCCH 2 CH 3HP+CCH 2 CH 3-HPCCH 2 CH 3 + CH 2 CH 3P C -b. Reactivity: The HEE are those in the C-P bond, which is polarized toward the carbon atom. Consequently Wittig reagents are carbonnucleophiles and phosphorus electrophiles.NOTE: Since P is in the Third Row of the Periodic Table, it has low energy d-orbitals that allow it to expand its octet and form4-membered ring transition-states and intermediate structures.O+OPP P O +The net effect of a Wittig reaction is an addition-elimination in which the carbon ligand on the phosphorus replaces the carbonyl oxygenatom. So a ketone is converted to an alkene.