Group theory
Group theory
Group theory
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
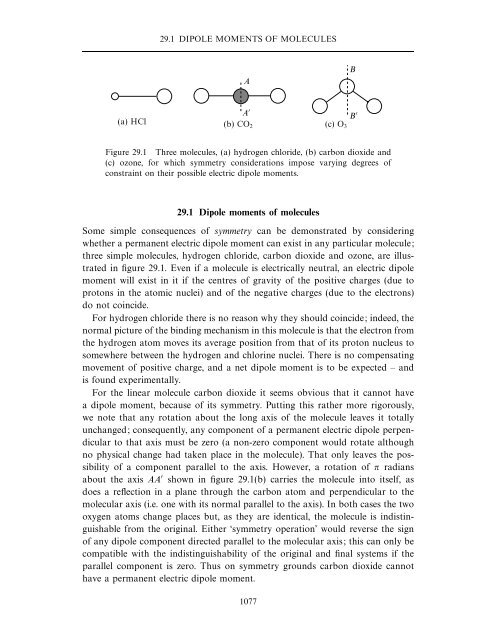
29.1 DIPOLE MOMENTS OF MOLECULESAB(a) HCl (b) CO 2 (c) O 3A B Figure 29.1 Three molecules, (a) hydrogen chloride, (b) carbon dioxide and(c) ozone, for which symmetry considerations impose varying degrees ofconstraint on their possible electric dipole moments.29.1 Dipole moments of moleculesSome simple consequences of symmetry can be demonstrated by consideringwhether a permanent electric dipole moment can exist in any particular molecule;three simple molecules, hydrogen chloride, carbon dioxide and ozone, are illustratedin figure 29.1. Even if a molecule is electrically neutral, an electric dipolemoment will exist in it if the centres of gravity of the positive charges (due toprotons in the atomic nuclei) and of the negative charges (due to the electrons)do not coincide.For hydrogen chloride there is no reason why they should coincide; indeed, thenormal picture of the binding mechanism in this molecule is that the electron fromthe hydrogen atom moves its average position from that of its proton nucleus tosomewhere between the hydrogen and chlorine nuclei. There is no compensatingmovement of positive charge, and a net dipole moment is to be expected – andis found experimentally.For the linear molecule carbon dioxide it seems obvious that it cannot havea dipole moment, because of its symmetry. Putting this rather more rigorously,we note that any rotation about the long axis of the molecule leaves it totallyunchanged; consequently, any component of a permanent electric dipole perpendicularto that axis must be zero (a non-zero component would rotate althoughno physical change had taken place in the molecule). That only leaves the possibilityof a component parallel to the axis. However, a rotation of π radiansabout the axis AA shown in figure 29.1(b) carries the molecule into itself, asdoes a reflection in a plane through the carbon atom and perpendicular to themolecular axis (i.e. one with its normal parallel to the axis). In both cases the twooxygen atoms change places but, as they are identical, the molecule is indistinguishablefrom the original. Either ‘symmetry operation’ would reverse the signof any dipole component directed parallel to the molecular axis; this can only becompatible with the indistinguishability of the original and final systems if theparallel component is zero. Thus on symmetry grounds carbon dioxide cannothave a permanent electric dipole moment.1077