Phase I/II and Phase II Clinical Trials of Axitinib for Selected Cancers ...
Phase I/II and Phase II Clinical Trials of Axitinib for Selected Cancers ...
Phase I/II and Phase II Clinical Trials of Axitinib for Selected Cancers ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
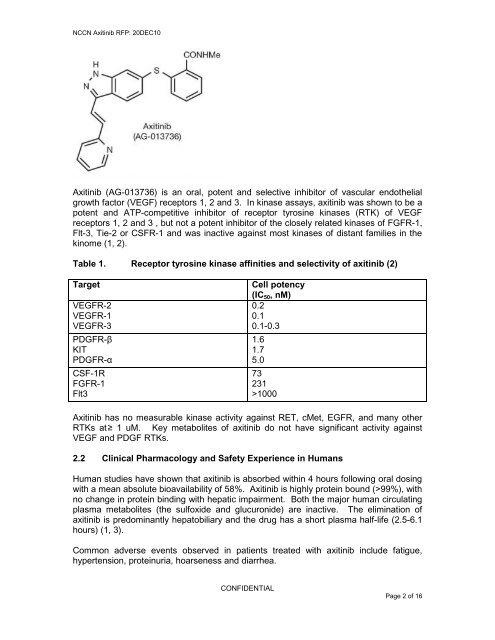
NCCN <strong>Axitinib</strong> RFP: 20DEC10<strong>Axitinib</strong> (AG-013736) is an oral, potent <strong>and</strong> selective inhibitor <strong>of</strong> vascular endothelialgrowth factor (VEGF) receptors 1, 2 <strong>and</strong> 3. In kinase assays, axitinib was shown to be apotent <strong>and</strong> ATP-competitive inhibitor <strong>of</strong> receptor tyrosine kinases (RTK) <strong>of</strong> VEGFreceptors 1, 2 <strong>and</strong> 3 , but not a potent inhibitor <strong>of</strong> the closely related kinases <strong>of</strong> FGFR-1,Flt-3, Tie-2 or CSFR-1 <strong>and</strong> was inactive against most kinases <strong>of</strong> distant families in thekinome (1, 2).Table 1. Receptor tyrosine kinase affinities <strong>and</strong> selectivity <strong>of</strong> axitinib (2)TargetVEGFR-2VEGFR-1VEGFR-3PDGFR-βKITPDGFR-αCSF-1RFGFR-1Flt3Cell potency(IC 50 , nM)0.20.10.1-0.31.61.75.073231>1000<strong>Axitinib</strong> has no measurable kinase activity against RET, cMet, EGFR, <strong>and</strong> many otherRTKs at ≥ 1 uM. Key metabolites <strong>of</strong> axitinib do not have significant activity againstVEGF <strong>and</strong> PDGF RTKs.2.2 <strong>Clinical</strong> Pharmacology <strong>and</strong> Safety Experience in HumansHuman studies have shown that axitinib is absorbed within 4 hours following oral dosingwith a mean absolute bioavailability <strong>of</strong> 58%. <strong>Axitinib</strong> is highly protein bound (>99%), withno change in protein binding with hepatic impairment. Both the major human circulatingplasma metabolites (the sulfoxide <strong>and</strong> glucuronide) are inactive. The elimination <strong>of</strong>axitinib is predominantly hepatobiliary <strong>and</strong> the drug has a short plasma half-life (2.5-6.1hours) (1, 3).Common adverse events observed in patients treated with axitinib include fatigue,hypertension, proteinuria, hoarseness <strong>and</strong> diarrhea.CONFIDENTIALPage 2 <strong>of</strong> 16