Lab Manual Appendix Activity Sheet - Moravian College Chemistry ...
Lab Manual Appendix Activity Sheet - Moravian College Chemistry ...
Lab Manual Appendix Activity Sheet - Moravian College Chemistry ...
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
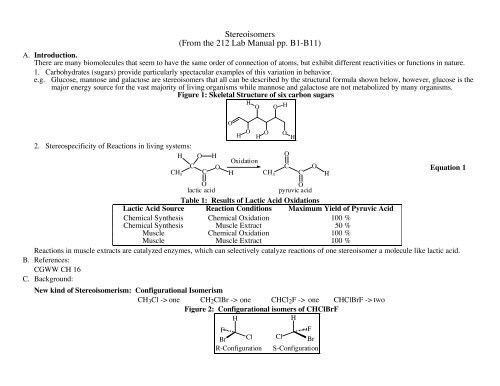
Stereoisomers(From the 212 <strong>Lab</strong> <strong>Manual</strong> pp. B1-B11)A. Introduction.There are many biomolecules that seem to have the same order of connection of atoms, but exhibit different reactivities or functions in nature.1. Carbohydrates (sugars) provide particularly spectacular examples of this variation in behavior.e.g. Glucose, mannose and galactose are stereoisomers that all can be described by the structural formula shown below, however, glucose is themajor energy source for the vast majority of living organisms while mannose and galactose are not metabolized by many organisms.Figure 1: Skeletal Structure of six carbon sugars2. Stereospecificity of Reactions in living systems:HOHCH 3CCOOHOlactic acidOpyruvic acidTable 1: Results of Lactic Acid OxidationsLactic Acid Source Reaction Conditions Maximum Yield of Pyruvic AcidChemical Synthesis Chemical Oxidation 100 %Chemical Synthesis Muscle Extract 50 %Muscle Chemical Oxidation 100 %Muscle Muscle Extract 100 %Reactions in muscle extracts are catalyzed enzymes, which can selectively catalyze reactions of one stereoisomer a molecule like lactic acid.B. References:CGWW CH 16C. Background:HOOHOOHOOOxidationCH CH 3New kind of Stereoisomerism: Configurational IsomerismCH 3 Cl -> one CH 2 ClBr -> one CHCl 2 F -> one CHClBrF -> twoFigure 2: Configurational isomers of CHClBrFHHFBr ClR-ConfigurationHCOFCl BrS-ConfigurationHEquation 1
2 StereoisomersConfigurational isomerism (optical isomerism) results from differences in the directions from one substituent on a carbon atom to another substituenton the same carbon atom (e.g. from the Br to the Cl in the structures above.). Molecules that can exist as two non-superimposable mirror imagestructures are known as chiral compounds. The term chiral is from Greek meaning handed. One cause of this type of isomerism is a carbon atomthat has four different substituents. Atoms of this type make up one kind of chiral atom (also known as centers of chirality, stereogenic atoms orstereocenters). Chiral atoms have configurations such that interchange of any two ligands produces a new stereoisomer. Recall thatinterchanging any two of the atoms of the R-isomer above produces its mirror image (S-isomer).D. Explorations:HHOOHCOCH 3R-ConfigurationO O HCHCH 3O HS-ConfigurationHOOOHglyceraldehydeOHOOHOH4 carbonsFigure 3: Lactic Acid Configurational IsomersFigure 4: 3 & 4 Carbon Carbohydrates1. Recognizing Chiral Atomsa. Lactic Acid (See Eq. 1) has one chiral atom illustrated in Figure 3 with wedges and dash-wedges. Thus it has two possible configurationalisomers. Note that the three carbon carbohydrate in Figure 4, glyceraldehyde, also has one chiral carbon atom and can exist as twoconfigurational isomers. Put a checkmark (√) on the chiral atom in the glyceraldehyde structure in Figure 4 and draw wedge dash-wedgestructures of its two configurational isomers.b. The four carbon carbohydrate in Figure 4 also has configurational isomers. How many chiral carbon atoms does the four carbon carbohydratehave? Indicate each chiral carbon atom with a checkmark (√).c. How many configurational isomers are possible for the four carbon carbohydrate structure in Figure 4? Explain your reasoning. Draw wedgedash-wedge structures for all of the configurational isomers of the four carbon carbohydrate.
Stereoisomers 32. Drawing Fischer Projections: See also CGWW p. 395.As you may have noted in devising structures in 1., when a structure can have multiple stereoisomers, representing them on paper can becumbersome. Fischer Projections are relatively simple two-dimensional representations of three-dimensional structures and are particularly usefulfor representing the configurations of atoms in molecules with multiple chiral atoms.Fischer Projections require that the molecule be oriented in a specific manner so that a flat representation (projection or shadow in twodimensions)can give three-dimensional information.Figure 5: Wedge Dash-Wedge (left) with corresponding Fisher Projection Structures (right)HBrBrCH 3HHClCH 2ClCH 2 CH 3H ClUsing the examples in Figure 5 as models, draw the Fischer Projection structure of:HOCH 3HHBrExplain the logic you used to determine your Fischer Projection.ClHCH 2 ClHCl HBr HCl CH 3CH Cl3HHClHCH 3ClCH 3HBrb. Using Fischer Projections:Figure 6 provides the Fischer Projections of D-glucose, D-mannose, and D-galactose as well as L-glucose.Figure 6: Fischer Projections of Some Six Carbon SugarsH 1 O H O H O H O2H OH HO H H OH HO H3HO H HO H HO H H OH4H OH H OH HO H HO HH5OH H OH H OH HO H6CH 2 OH CH 2 OH CH 2 OH CH 2 OHD-glucose D-mannose D-galactose L-glucose(1.) How many chiral atoms are there in each of these structures in Figure 6? Using the numbering system illustrated for D-glucose, indicatewhich atoms are chiral. How did you recognize them?(2.) Using the numbering system illustrated for D-glucose, explain how the four structures differ.
4 Stereoisomers3. Defining and Recognizing Relationships Among Configurational Stereoisomers:Explorations of terms that define relationships among configurational isomers: ENANTIOMERS, DIASTEREOMERS & EPIMERS Seealso CGWW pp. 382-396a. ENANTIOMERS:Figure 7: Examples of ENANTIOMERS (Enantiomeric Pairs of Compounds):These pairs of stereoisomers areENANTIOMERSH OH H OHThese pairs of stereoisomers are NOTENANTIOMERSH OH HO H1 2H CH 3CH 3CH 3 H H CH 33 4CH 3 CH 3Cl H H Cl&H Cl Cl HCH 3 CH 35 6CH 3 CH 3HO H&H OHH OH HO HCH 2 CH 3 CH 2 CH 37 8(1.) Place a checkmark (√) next to all chiral atoms in Figure 7.H9 10H CH H 3HCH 3 H CH 3CH 3Cl HH ClCH 3CH 3HO HHOHCH 2 CH 311 12&CH 3Cl HCl HCH 313 14&CH 3HO HHOHCH 2 CH 315 16CH 3(2.) How are the configurations of the chiral atoms of the enantiomeric stereoisomers in Figure 7 related to each other? Explain how youdiscerned the relationships.(3.) How are the configurations of the chiral atoms of non-enantiomeric stereoisomers in Figure 7 related to each other? Explain how youdiscerned the relationships.(4.) Definition: On the basis of the structures in Figure 7, define, as specifically as possible, the relationship between the members of apair of enantiomers. Explain the logic used to devise your definition.
Stereoisomers 7(1.) Place a checkmark (√) next to all chiral atoms in Figure 10.(2.) How are the configurations of the chiral atoms of the epimeric stereoisomers in Figure 10 related to each other? Explain how youdiscerned the relationships.(3.) How are the configurations of the chiral atoms of the non-epimeric stereoisomers in Figure 10 related to each other? Explain howyou discerned the relationships.(4.) Definition: On the basis of the structures in Figure 10, define, as specifically as possible, the relationship between stereoisomers thatare epimers. Explain the logic used to devise your definition.(5.) Application: Identify all of the all pairs of compounds in Figure 11 that are epimers. Briefly explain the logic of your classifications.Figure 11: Structures for epimer problems.(a.)(b.)(c.)CH 3 CH 3HO OHO H H OH H OHHCHCl H OH H Cl2 OH&HO H HO HH OH H OHHO H H OHCH 2 CH 3 CH 2 CHH OH H OH3HO HH OH H OH H OHCH 2 OHHO O
8 Stereoisomers4. Defining and Recognizing Types of Stereoisomersa. CHIRAL COMPOUNDS:Figure 12: Examples of CHIRAL and ACHIRAL (not chiral) CompoundsThese compounds are CHIRAL These compounds are NOT CHIRAL(achiral)H OHHO HAECH 3 HH HBH CHF3CHCH 3CH 33CHCl H3CCl HH ClGCl HCH 3CH 3CH 3CHHO H2 CH 3DHO HHO HHHO HCH 2 CH 3CH 2 CH 3(1.) Place a checkmark (√) next to all chiral atoms in Figure 12.(2.) Do all chiral molecules in Figure 12 contain chiral atoms?(3.) Do any achiral molecules in Figure 12 contain chiral atoms? If so which ones?(4.) Definition: On the basis of the structures in Figure 12, define, as specifically as possible, the characteristics required for a compoundto be CHIRAL. Explain the logic used to devise your definition.
Stereoisomers 9(5.) Application: Using the definition you derived in (4), identify all of the chiral compounds in the Figure 13. Briefly explain the logicof your classifications.Figure 13: Structures for chiral and meso molecule problems.(a.)(b.)(c.)(d.)H OHO OCH OHCHH OH2 OHH OHHO H HO HCH 2 OHHO H HO HH OHCH 2 OHCH 2 OHb. MESO COMPOUNDS:These compounds are MESOH HI CH 3CH 3CH 3Cl HCl HJCH 3Figure 14: Examples of MESO COMPOUNDSThese compounds are NOT MESOHO HLCH 3 HM H CH 3CH 3Cl HH ClNCH 3CH 3 CH 3HO HHO HH OHHO HHO HPCH 2 CH 3KCH 3(1.) Place a checkmark (√) next to all chiral atoms in Figure 14.(2.) Circle all chiral molecules in Figure 14.(3.) Is any MESO compound in Figure 14 chiral? If so, which one(s)?
10 Stereoisomers(4.) Definition: On the basis of the structures in Figure 14, define, as specifically as possible, the characteristics required for a compoundto be MESO. Explain the logic used to devise your definition.(5.) Application: Identify all of the MESO compounds in Figure 13. Briefly explain the logic of your classifications.1. Draw the required structure in each item below and briefly explain how your structure fulfills the requirements of the item:a.Enantiomer ofb.Diastereomer ofc.C3 epimer ofHO OH OHHO HHO HH OHCH 2 OHHOHOCH 3HHCH 2 CH 3HO OH OHHO HHO HH OHCH 2 OHd.Meso diastereomer ofd.Chiral diastereomer ofe.Enantiomer ofHOHHCH 3HOHOHCH 3HHOHOHCH 2 OHOHHHOHCH 2 OHHBrOHH2. Is it possible for a molecule to have more than one enantiomer? Explain using an example.3. Is it possible for a molecule to have more than one diastereomer? Explain using an example.4. Is it possible for a molecule to have more than one epimer? Explain using an example.
Stereoisomers 115. Is it possible for a molecule to be chiral and meso? Explain using an example.6. Is it possible for a molecule with one chiral atom to be a meso compound? Explain using an example.F. Nomenclature of Chiral compounds1. CGWW: pp. 387-3882. Tutorials:References:http://www.cem.msu.edu/~reusch/VirtualText/sterism3.htm#isom13Provides naming rules and examplesDeveloped at Michigan State Universityhttp://www.molecularmodels.ca/nomenclature/index-2.htmDeveloped by Professor Dave Woodcock,Okanagan University <strong>College</strong>, British Columbia, CanadaSections:7. Stereochemistry (iii) ChiralityIV. Naming Enantiomers: R/S System(i) One Chirality Centre(ii) More than One Chirality Centre(Contains many examples.)Note: Your browser must have the chemscape chime plug-in for these pages to work. Select "1. Introduction to these pages", thenclick on "Nomenclature Index - Chemscape Chime" and begin with the "How and Why". If you have problems, go to thehttp://www.molecularmodels.ca/nomenclature/nom1.htm and click on "chemscape chime" and follow directions to download the"chemscape chime plug-in." If you have problems contact me.
12 Stereoisomersb. http://www.acdlabs.com/iupac/nomenclatureDeveloped by Advanced <strong>Chemistry</strong> Development <strong>Lab</strong>oratories(Gives detailed IUPAC rules for nomenclature.)Recommendations 1993R-7 Stereochemical SpecificationR-7.2 Chiral Compounds Specification of Absolute ConfigurationR-7.2.1 The R/S conventionc. Out of Class Applications of Nomenclature.HOBrO(1) Name the following:HH CH 3 H OHHCH 3ClHHOHHO CH 3(2) Draw structural formulas for the following compounds:R 2-iodobutaneS ethyl 2-hydroxypentanoate(2S,3R) 3-methyl-2-pentanolR 2-bromobutanoic acid