Available Online through www.ijptonline.com
Available Online through www.ijptonline.com
Available Online through www.ijptonline.com
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
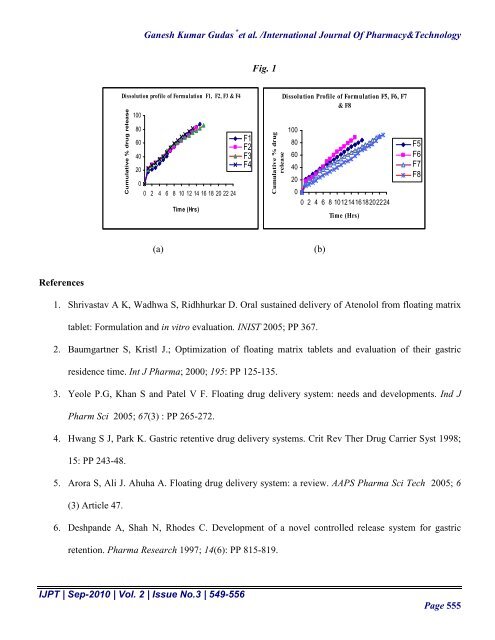
Ganesh Kumar Gudas * et al. /International Journal Of Pharmacy&TechnologyFig. 1Dissolution profile of Formulation F1, F2, F3 & F4Cumulative % drug release1008060402000 2 4 6 8 10 12 14 16 18 20 22 24Time (Hrs)F1F2F3F4Cumulative % drugreleaseDissolution Profile of Formulation F5, F6, F7& F81008060402000 2 4 6 8 1012141618202224Time (Hrs)F5F6F7F8(a)(b)References1. Shrivastav A K, Wadhwa S, Ridhhurkar D. Oral sustained delivery of Atenolol from floating matrixtablet: Formulation and in vitro evaluation. INIST 2005; PP 367.2. Baumgartner S, Kristl J.; Optimization of floating matrix tablets and evaluation of their gastricresidence time. Int J Pharma; 2000; 195: PP 125-135.3. Yeole P.G, Khan S and Patel V F. Floating drug delivery system: needs and developments. Ind JPharm Sci 2005; 67(3) : PP 265-272.4. Hwang S J, Park K. Gastric retentive drug delivery systems. Crit Rev Ther Drug Carrier Syst 1998;15: PP 243-48.5. Arora S, Ali J. Ahuha A. Floating drug delivery system: a review. AAPS Pharma Sci Tech 2005; 6(3) Article 47.6. Deshpande A, Shah N, Rhodes C. Development of a novel controlled release system for gastricretention. Pharma Research 1997; 14(6): PP 815-819.IJPT | Sep-2010 | Vol. 2 | Issue No.3 | 549-556Page 555