(bioequivalence) requirements - BEBAC ⢠Consultancy Services for ...
(bioequivalence) requirements - BEBAC ⢠Consultancy Services for ...
(bioequivalence) requirements - BEBAC ⢠Consultancy Services for ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
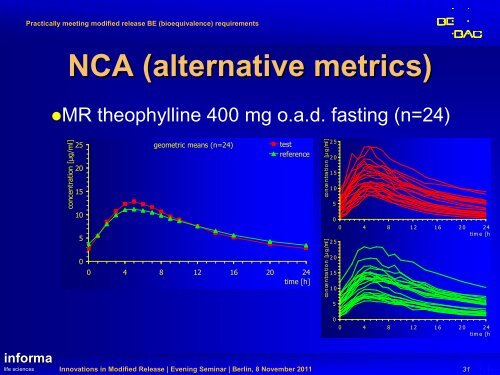
Practically meeting modified release BE (<strong>bioequivalence</strong>) <strong>requirements</strong>NCA (alternative metrics)MR theophylline 400 mg o.a.d. fasting (n=24)concentration [µg/ml]2520151050geometric means (n=24)testreference0 4 8 12 16 20 24time [h]co n ce n tra tio n [µ g /ml ]co n ce n tra tio n [µ g /ml ]2 52 01 51 0502 52 01 51 050 4 8 1 2 1 6 2 0 2 4ti m e [h ]00 4 8 1 2 1 6 2 0 2 4ti m e [h ]in<strong>for</strong>malife sciencesInnovations in Modified Release | Evening Seminar | Berlin, 8 November 201131