Focus 5.10 Reactions
Focus 5.10 Reactions
Focus 5.10 Reactions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
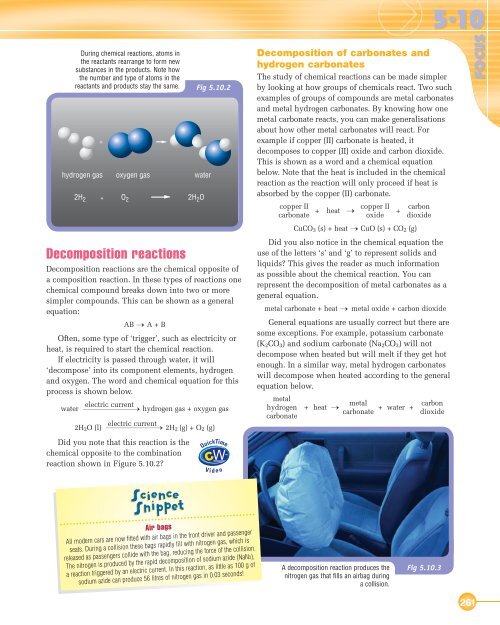
During chemical reactions, atoms inthe reactants rearrange to form newsubstances in the products. Note howthe number and type of atoms in thereactants and products stay the same. Decomposition reactionsDecomposition reactions are the chemical opposite ofa composition reaction. In these types of reactions onechemical compound breaks down into two or moresimpler compounds. This can be shown as a generalequation:AB → A + BOften, some type of ‘trigger’, such as electricity orheat, is required to start the chemical reaction.If electricity is passed through water, it will‘decompose’ into its component elements, hydrogenand oxygen. The word and chemical equation for thisprocess is shown below.electric currentwaterhydrogen gas + oxygen gas2H 2 O (l)electric currentDid you note that this reaction is thechemical opposite to the combinationreaction shown in Figure <strong>5.10</strong>.2?Fig <strong>5.10</strong>.22H 2 (g) + O 2 (g)Decomposition of carbonates andhydrogen carbonatesThe study of chemical reactions can be made simplerby looking at how groups of chemicals react. Two suchexamples of groups of compounds are metal carbonatesand metal hydrogen carbonates. By knowing how onemetal carbonate reacts, you can make generalisationsabout how other metal carbonates will react. Forexample if copper (II) carbonate is heated, itdecomposes to copper (II) oxide and carbon dioxide.This is shown as a word and a chemical equationbelow. Note that the heat is included in the chemicalreaction as the reaction will only proceed if heat isabsorbed by the copper (II) carbonate.copper IIcarbonate + heat →copper IIoxide+ carbondioxideCuCO 3 (s) + heat → CuO (s) + CO 2 (g)Did you also notice in the chemical equation theuse of the letters ‘s’ and ‘g’ to represent solids andliquids? This gives the reader as much informationas possible about the chemical reaction. You canrepresent the decomposition of metal carbonates as ageneral equation.metal carbonate + heat → metal oxide + carbon dioxideGeneral equations are usually correct but there aresome exceptions. For example, potassium carbonate(K 2 CO 3 ) and sodium carbonate (Na 2 CO 3 ) will notdecompose when heated but will melt if they get hotenough. In a similar way, metal hydrogen carbonateswill decompose when heated according to the generalequation below.metalhydrogencarbonate+ heat → metalcarbonate + water +<strong>5.10</strong>carbondioxideFOCUSAir bagsAll modern cars are now fitted with air bags in the front driver and passengerseats. During a collision these bags rapidly fill with nitrogen gas, which isreleased as passengers collide with the bag, reducing the force of the collision.The nitrogen is produced by the rapid decomposition of sodium azide (NaN3),a reaction triggered by an electric current. In this reaction, as little as 100 g ofsodium azide can produce 56 litres of nitrogen gas in 0.03 seconds!A decomposition reaction produces thenitrogen gas that fills an airbag duringa collision.Fig <strong>5.10</strong>.3261