Effects of Low-Purge Vehicle Applications and ... - MeadWestvaco
Effects of Low-Purge Vehicle Applications and ... - MeadWestvaco
Effects of Low-Purge Vehicle Applications and ... - MeadWestvaco
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
portion <strong>of</strong> the canister carbon bed closest to the<br />
atmosphere port.<br />
EFFECT OF CARBON HONEYCOMB ON CANISTER<br />
WORKING CAPACITY<br />
As a result <strong>of</strong> testing for canister bleed emissions<br />
performance, it was discovered that activated carbon<br />
honeycombs have a significant positive impact on<br />
diurnal canister working capacity. The effect <strong>of</strong> the<br />
activated carbon honeycomb on canister gasoline<br />
working capacity is summarized in Figure 7.<br />
Canister G W C (g) .<br />
180<br />
160<br />
140<br />
120<br />
100<br />
80<br />
60<br />
40<br />
20<br />
0<br />
+4%<br />
+11%<br />
+13%<br />
+19%<br />
No HCA 29x100-200 41x150-200 50x150-200 Heated<br />
41x150<br />
Figure 7. Effect <strong>of</strong> activated carbon honeycomb on<br />
canister working capacity.<br />
The baseline gasoline working capacity <strong>of</strong> 138 g<br />
presented in Figure 6 was obtained with a 2.1-liter<br />
canister filled with BAX 1500 without an activated carbon<br />
honeycomb attached. An increase in overall canister<br />
working capacity <strong>of</strong> 4% to 19% was demonstrated with<br />
the addition <strong>of</strong> an activated carbon honeycomb. The<br />
increased capacity was proportional to the honeycomb<br />
size <strong>and</strong> therefore the capacity <strong>of</strong> the honeycomb to<br />
adsorb hydrocarbon vapors.<br />
EFFECT OF ETHANOL FUEL BLENDS ON CARBON<br />
BED PERFORMANCE<br />
Adsorption <strong>of</strong> Ethanol<br />
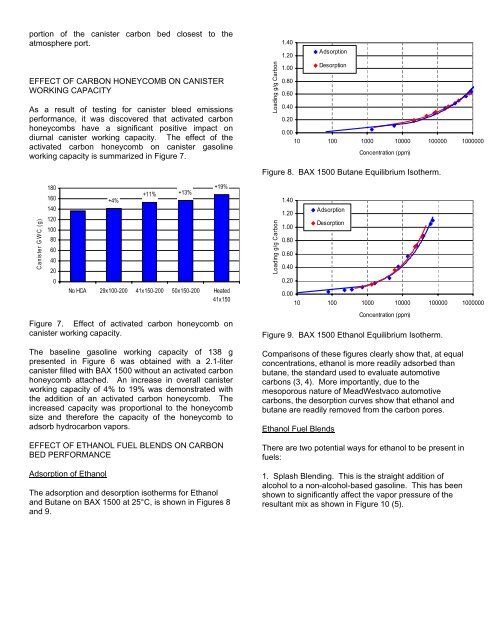
The adsorption <strong>and</strong> desorption isotherms for Ethanol<br />
<strong>and</strong> Butane on BAX 1500 at 25°C, is shown in Figures 8<br />
<strong>and</strong> 9.<br />
Loading g/g Carbon<br />
1.40<br />
1.20<br />
1.00<br />
0.80<br />
0.60<br />
0.40<br />
0.20<br />
Adsorption<br />
Desorption<br />
0.00<br />
10 100 1000 10000 100000 1000000<br />
Concentration (ppm)<br />
Figure 8. BAX 1500 Butane Equilibrium Isotherm.<br />
Loading g/g Carbon<br />
1.40<br />
1.20<br />
1.00<br />
0.80<br />
0.60<br />
0.40<br />
0.20<br />
Adsorption<br />
Desorption<br />
0.00<br />
10 100 1000 10000 100000 1000000<br />
Concentration (ppm)<br />
Figure 9. BAX 1500 Ethanol Equilibrium Isotherm.<br />
Comparisons <strong>of</strong> these figures clearly show that, at equal<br />
concentrations, ethanol is more readily adsorbed than<br />
butane, the st<strong>and</strong>ard used to evaluate automotive<br />
carbons (3, 4). More importantly, due to the<br />
mesoporous nature <strong>of</strong> <strong>MeadWestvaco</strong> automotive<br />
carbons, the desorption curves show that ethanol <strong>and</strong><br />
butane are readily removed from the carbon pores.<br />
Ethanol Fuel Blends<br />
There are two potential ways for ethanol to be present in<br />
fuels:<br />
1. Splash Blending. This is the straight addition <strong>of</strong><br />
alcohol to a non-alcohol-based gasoline. This has been<br />
shown to significantly affect the vapor pressure <strong>of</strong> the<br />
resultant mix as shown in Figure 10 (5).