Study Overview - Clinical Trial Results .org
Study Overview - Clinical Trial Results .org
Study Overview - Clinical Trial Results .org
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
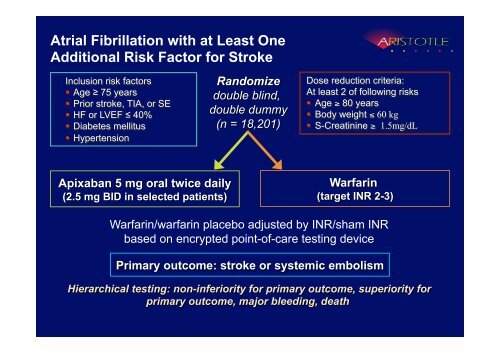
Atrial Fibrillation with at Least OneAdditional Risk Factor for StrokeInclusion risk factors Age ≥ 75 years Prior stroke, TIA, or SE HF or LVEF ≤ 40% Diabetes mellitus HypertensionRandomizedouble blind,double dummy(n = 18,201)Dose reduction criteria:At least 2 of following risks Age ≥ 80 years Body weight ≤ 60 kg S-Creatinine ≥ 1.5mg/dLApixaban 5 mg oral twice daily(2.5 mg BID in selected patients)Warfarin(target INR 2-3)Warfarin/warfarin placebo adjusted by INR/sham INRbased on encrypted point-of-care testing devicePrimary outcome: stroke or systemic embolismHierarchical testing: non-inferiority for primary outcome, superiority forprimary outcome, major bleeding, death