View - ResearchGate
View - ResearchGate
View - ResearchGate
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
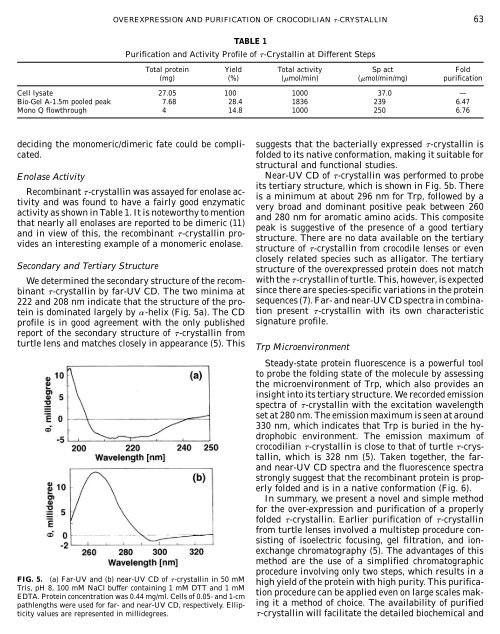
OVEREXPRESSION AND PURIFICATION OF CROCODILIAN -CRYSTALLIN 63TABLE 1Purification and Activity Profile of -Crystallin at Different StepsTotal protein Yield Total activity Sp act Fold(mg) (%) (mol/min) (mol/min/mg) purificationCell lysate 27.05 100 1000 37.0 —Bio-Gel A-1.5m pooled peak 7.68 28.4 1836 239 6.47Mono Q flowthrough 4 14.8 1000 250 6.76deciding the monomeric/dimeric fate could be complicated.Enolase ActivitySecondary and Tertiary StructureWe determined the secondary structure of the recom-binant -crystallin by far-UV CD. The two minima at222 and 208 nm indicate that the structure of the pro-tein is dominated largely by -helix (Fig. 5a). The CDprofile is in good agreement with the only publishedreport of the secondary structure of -crystallin fromturtle lens and matches closely in appearance (5). ThisRecombinant -crystallin was assayed for enolase ac-tivity and was found to have a fairly good enzymaticactivity as shown in Table 1. It is noteworthy to mentionthat nearly all enolases are reported to be dimeric (11)and in view of this, the recombinant -crystallin pro-vides an interesting example of a monomeric enolase.suggests that the bacterially expressed -crystallin isfolded to its native conformation, making it suitable forstructural and functional studies.Near-UV CD of -crystallin was performed to probeits tertiary structure, which is shown in Fig. 5b. Thereis a minimum at about 296 nm for Trp, followed by avery broad and dominant positive peak between 260and 280 nm for aromatic amino acids. This compositepeak is suggestive of the presence of a good tertiarystructure. There are no data available on the tertiarystructure of -crystallin from crocodile lenses or evenclosely related species such as alligator. The tertiarystructure of the overexpressed protein does not matchwith the -crystallin of turtle. This, however, is expectedsince there are species-specific variations in the proteinsequences (7). Far- and near-UV CD spectra in combinationpresent -crystallin with its own characteristicsignature profile.Trp MicroenvironmentSteady-state protein fluorescence is a powerful toolto probe the folding state of the molecule by assessingthe microenvironment of Trp, which also provides aninsight into its tertiary structure. We recorded emissionspectra of -crystallin with the excitation wavelengthset at 280 nm. The emission maximum is seen at around330 nm, which indicates that Trp is buried in the hydrophobicenvironment. The emission maximum ofcrocodilian -crystallin is close to that of turtle -crystallin,which is 328 nm (5). Taken together, the farandnear-UV CD spectra and the fluorescence spectrastrongly suggest that the recombinant protein is properlyfolded and is in a native conformation (Fig. 6).In summary, we present a novel and simple methodfor the over-expression and purification of a properlyfolded -crystallin. Earlier purification of -crystallinfrom turtle lenses involved a multistep procedure consistingof isoelectric focusing, gel filtration, and ionexchangechromatography (5). The advantages of thismethod are the use of a simplified chromatographicprocedure involving only two steps, which results in aFIG. 5. (a) Far-UV and (b) near-UV CD of -crystallin in 50 mM high yield of the protein with high purity. This purifica-Tris, pH 8, 100 mM NaCl buffer containing 1 mM DTT and 1 mM tion procedure can be applied even on large scales mak-EDTA. Protein concentration was 0.44 mg/ml. Cells of 0.05- and 1-cmpathlengths were used for far- and near-UV CD, respectively. Ellipticityvalues are represented in millidegrees.ing it a method of choice. The availability of purified-crystallin will facilitate the detailed biochemical and